Synthetic method of bepidic acid
A synthesis method and compound technology, which is applied in the field of synthesis of the compound bempedelic acid, can solve problems such as unfavorable raw material drug quality control, difficulty in industrial production, poor atom economy, etc., and achieve green production process, simplified operation, and reduced dosage Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] (1) Preparation of compound 1: In a 250mL two-necked flask, the nitrogen was replaced three times, then ethyl isobutyrate (4.2g, 36mmol) and 40mL tetrahydrofuran were added, stirred at -40°C for 10 minutes, and then diisopropyl was added dropwise Lithium amide (18mL, 36mmol), reacted for 1 hour, added 1,5-dibromopentane (11.8g, 51.6mmol) dropwise, reacted for 0.5 hour, moved to room temperature for 8 hours, and then used 15mL ice Quenched with water, extracted three times with ethyl acetate, combined the organic phases, dried over anhydrous sodium sulfate, concentrated to dryness, and passed through the column to obtain compound 1 (7.2 g, 76%).
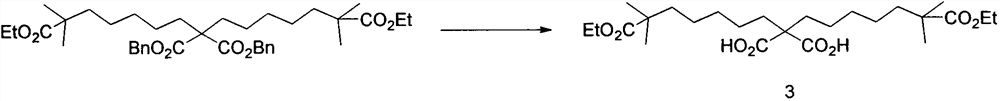
[0036] (2) Preparation of compound 2: Add dibenzyl malonate (1.42g, 5mmol), 15mL toluene and 15mL dimethylformamide into a 100mL single-necked bottle, stir for 5 minutes in an ice bath, and then add sodium hydride in batches (360mg, 12mmol, 60%), after reacting for 1 hour, compound 1 (2.78g, 10.5mmol) was added, and the tempera...
Embodiment 2
[0042] (1) Preparation of compound 1: In a 250mL two-necked flask, the nitrogen was replaced three times, then ethyl isobutyrate (4.2g, 36mmol) and 40mL tetrahydrofuran were added, stirred at -40°C for 10 minutes, and then diisopropyl was added dropwise Lithium amide (18mL, 36mmol), reacted for 1 hour, added 1,5-dibromopentane (9.2g, 40.0mmol) dropwise, reacted for 0.5 hour, moved to room temperature for 8 hours, and then used 15mL ice Quenched with water, extracted three times with ethyl acetate, combined the organic phases, dried over anhydrous sodium sulfate, concentrated to dryness, and passed through the column to obtain compound 1 (6.5 g, 69%).
[0043] (2) Preparation of compound 2: Add dibenzyl malonate (1.42g, 5mmol), 15mL toluene and 15mL dimethylformamide into a 100mL single-necked bottle, stir for 5 minutes in an ice bath, and then add sodium hydride in batches (180mg, 6mmol, 60%), after reacting for 1 hour, compound 1 (1.32g, 5mmol) was added, and the temperature ...
Embodiment 3
[0049] (1) Preparation of compound 1: In a 250mL two-necked flask, the nitrogen was replaced three times, then ethyl isobutyrate (4.2g, 36mmol) and 40mL tetrahydrofuran were added, stirred at -40°C for 10 minutes, and then diisopropyl was added dropwise Lithium amide (18mL, 36mmol), reacted for 1 hour, added 1,5-dibromopentane (12.5g, 54.5mmol) dropwise, reacted for 0.5 hour, moved to room temperature and reacted for 8 hours, and then used 15mL ice Quenched with water, extracted three times with ethyl acetate, combined the organic phases, dried over anhydrous sodium sulfate, concentrated to dryness, and passed through the column to obtain compound 1 (6.9 g, 73%).
[0050] (2) Preparation of compound 2: Add dibenzyl malonate (1.42g, 5mmol), 15mL toluene and 15mL dimethylformamide into a 100mL single-necked bottle, stir for 5 minutes in an ice bath, and then add sodium hydride in batches (375mg, 12.5mmol, 60%), after reacting for 1 hour, compound 1 (3.31g, 12.5mmol) was added, h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com