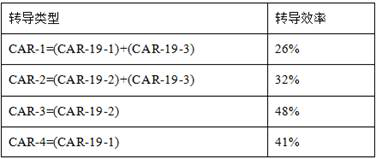
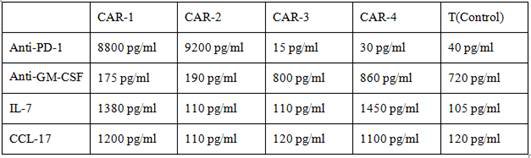
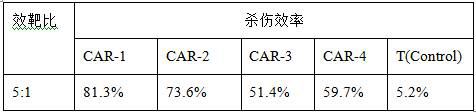
Method for preparing CAR-T cells
A technology of cells and culture medium, which is applied in the field of biotechnology and achieves the effect of excellent technical effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Example 1: Construction of recombinant plasmids
[0068] 1. Synthesis of CAR-19-1 plasmid:
[0069] The CAR-19-1 sequence is composed of CD19 single-chain variable region, CD8a hinge region, CD8a transmembrane region, 4-1BB signal, CD3ζ patina signal, IL-7, CCL17 sequence, and the vector used is pLVX-EF1a- IRES-PGK-puro, synthesized by General Biosystems (Anhui) Co., Ltd.
[0070] The nucleotide sequence of CAR-19-1 is as follows (may be referred to as sequence 1 in the present invention):
[0071]atggccctgcccgtgaccgctctgctgctgccactggccctgctgctgcacgccgctagacctgaggtgaagctgcaggagtccggccctggcctggtggctccttcccagtccctgagcgtgacctgtacagtgtccggcgtgtccctgcctgattacggcgtgtcctggatcaggcagcctcccagaaagggcctggagtggctgggcgtgatctggggctccgagacaacctactacaattccgccctgaagtccaggctgacaatcatcaaggacaatagcaagagccaggtgtttctgaagatgaactccctgcagacagacgacaccgccatctactactgcgccaagcactactactacggcggctcctacgccatggattactggggccagggcaccagcgtgacagtgtcctccggcggcggcggaagcggaggaggaggatctggcggcggcggttccgatatccagatg...
Embodiment 2
[0086] Example 2: Viral packaging
[0087] Invitrogen Lipofectamine 3000 transfection reagent (Lip3000) was used for virus packaging, and the experimental operation was basically performed according to the instructions of the transfection reagent.
[0088] 1. 293T medium configuration
[0089] The medium was DMEM-H supplemented with 10% FBS. (DMEM-H is the DMEM medium supplemented with 4.5g / L glucose and 110mg / L sodium pyruvate).
[0090] 2. Lentivirus packaging medium configuration:
[0091] The medium is Opti-MEMI, add 1% GlutaMAX, add sodium pyruvate (1mM), 5% FBS. (The Opti-MEMI medium is a Gibco product).
[0092] 3. Lentivirus packaging:
[0093] A) 293T cells were treated with 7x10 6 Cells / well were seeded in a 10cm culture dish containing 12 mL of lentiviral packaging medium, placed at 37°C, 5% CO 2 Incubate the cells overnight.
[0094] B) Transfect when the 293T cell density reaches 95%, the transfection method is:
[0095] Solution A configuration: First, r...
Embodiment 3
[0115] Example 3: T cell preparation
[0116] 1. Preparation of T cells
[0117] A) Add antibody (20 μL, CD8 biotin antibody, biotin-labeled CD8 antibody, BD product, Cat. No. 555365) to the EP tube containing 1 mL of whole blood, and mix for 30 minutes at room temperature with a rotator. The whole blood used in the experiment was taken from 8 healthy male volunteers (26-33 years old, body weight 58-71kg), and T cells were prepared respectively. The above-mentioned biotin-labeled CD8 antibody is expensive and is an important cost item in the method for preparing T cells of the present invention.
[0118] B) Add 150 μL of microbubbles (streptavidin, streptavidin-labeled, ThermoGenesis product) to the above 1 mL of whole blood, and mix with a rotator for 20 minutes at room temperature to combine the microbubbles with the antibody-labeled cells.
[0119] C) Centrifuge cells at 400 x g for 5 minutes.
[0120] D) Use a 200µL pipette gun to gently transfer the white microbubble l...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com