Artificially modified beta-galactosidase GaLT1 and application thereof in lactose hydrolysis
A technology for galactosidase and lactose hydrolysis, which is applied to beta-galactosidase GaLT1 and its application in lactose hydrolysis, and can solve problems such as poor stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Sequence design and synthesis of artificially modified β-galactosidase GaLT1
[0024] (1) Amphiphilic short peptide design
[0025] The laboratory designed a total of 8 parental short peptides:
[0026] ①AEAEAKAKAEAEAKAKAEAEAKAK;
[0027] ②AEAEAKAKAEAEAKAK;
[0028] ③LELELKLKLELELKLK;
[0029] ④ADADAKAKADADAKAKA;
[0030] ⑤ADADARARADADARAR;
[0031] ⑥AEAEAHAHAEAEAHAH;
[0032] ⑦HNANARARHNANARARHNANARARHNANARAR;
[0033] ⑧ANANARARANANARAR.
[0034] (2) The fusion of the amphipathic short peptide and the wild-type β-galactosidase (named GaLTO) derived from T. scotoductus. The amino acid sequence of the wild-type β-galactosidase GaLT0 is derived from the whole genome sequencing of T. scotoductus strain, the number in the NCBI database is WP_015716994.1, and it is annotated as α-amylase (alpha-amylase). There is no literature report on the related research of this protein. Entrusted Shanghai Sangon Biological Co., Ltd. to fuse and connect the designed 8...
Embodiment 2
[0035] Example 2: Recombinant expression and protein purification of artificially modified β-galactosidase GaLT1
[0036] 1. The genes of β-galactosidases fused with different parental short peptides were respectively cloned into pET22b, and then transferred into Escherichia coli BL21(DE3) strains to induce expression according to the standard procedure of molecular cloning. The obtained bacterial cells are homogeneously crushed under high pressure, and the centrifuged supernatant is crude enzyme liquid.
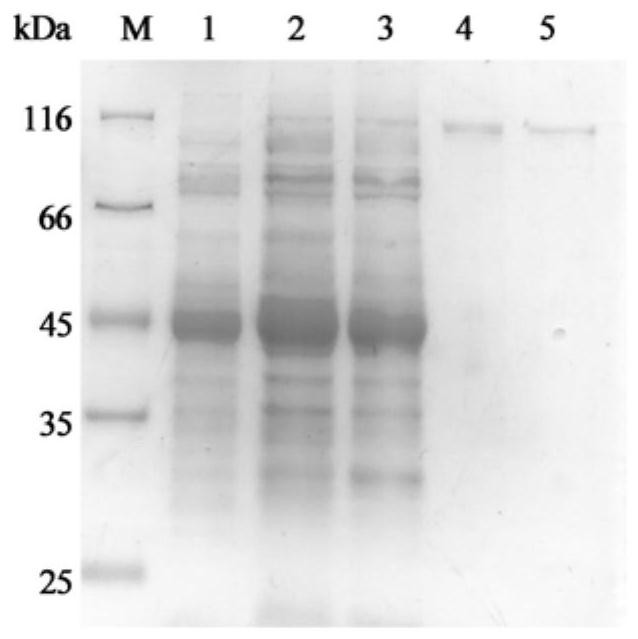
[0037] 2. The crude enzyme solution is purified by anion exchange chromatography to obtain artificially modified β-galactosidase recombinant protein. Protein purity was checked by SDS-PAGE. After routine enzyme activity testing, GaLT1 with the best comprehensive catalytic ability was screened out from 8 artificially modified β-galactosidases. The recombinant expression and protein purification of the enzyme are as follows: figure 1shown. M is a high molecular weight stan...
Embodiment 3
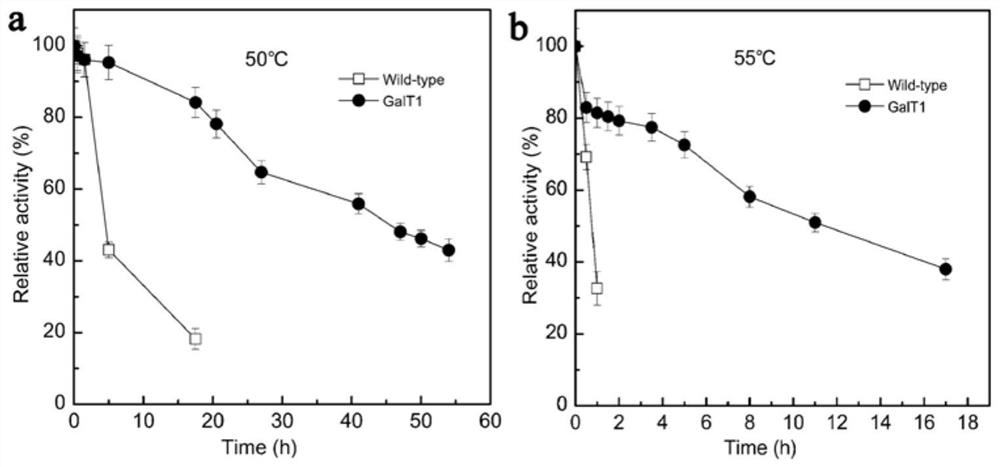
[0038] Embodiment 3: the optimal reaction temperature of artificially transformed β-galactosidase GaLT1 and wild-type enzyme GaLT0
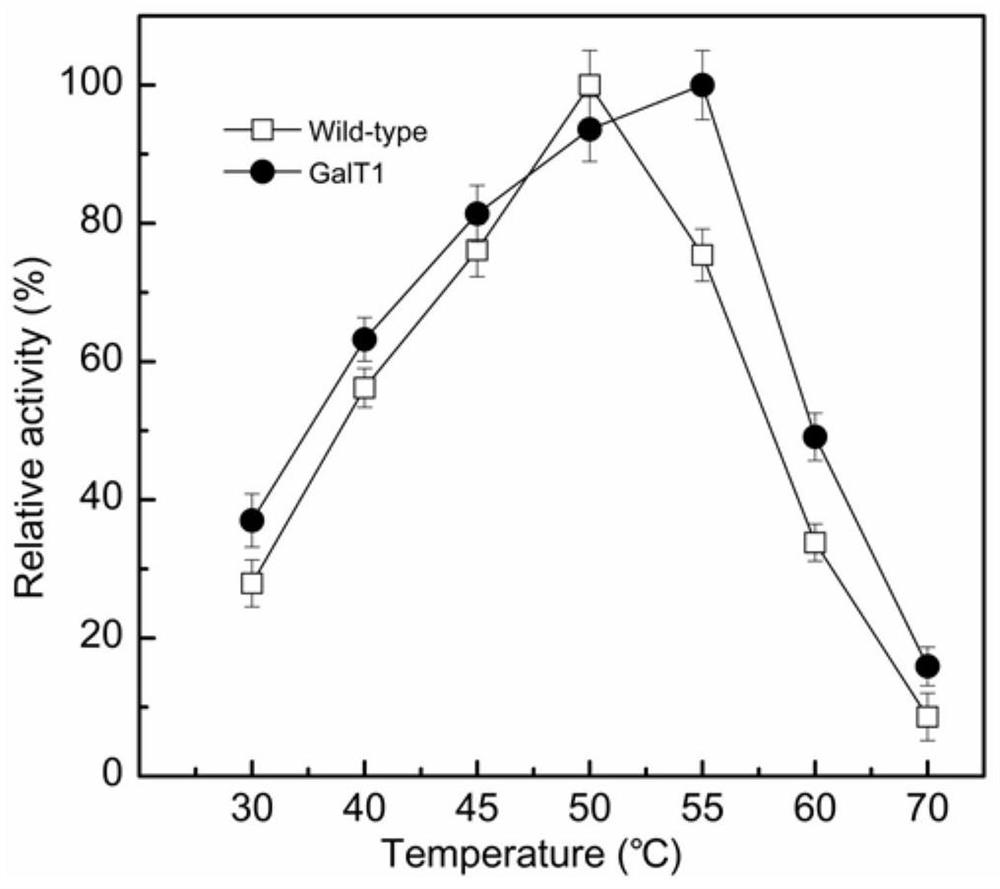
[0039] The enzyme reaction system was 500 μL, including 100 μL of 10 mM oNPG, 5 μL of appropriately diluted pure enzyme solution, and 395 μL of 50 mM phosphate buffer (pH 7.0). Carry out the reaction at 30-70°C for 10 minutes, and use the same volume of 1M Na at the end of the reaction 2 CO 3 The reaction was terminated, and the absorbance was measured at 405 nm. Such as figure 2 As shown, the optimum reaction temperature of the wild-type β-galactosidase GaLT0 is 50°C, and the optimum reaction temperature of the artificially modified β-galactosidase GaLT1 is increased to 55°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com