Method for monitoring and analyzing process of preparing 1, 3-cyclohexanedione through hydrogenation of resorcinol
A technology of cyclohexanedione and resorcinol, which is applied in the field of monitoring and analysis of the process of preparing 1,3-cyclohexanedione by hydrogenation of resorcinol, can solve problems such as insufficient analysis accuracy, and achieve good peak shape , Good repeatability and good peak shape
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Resorcinol hydrogenation reaction process is as follows:
[0058] Put resorcinol, sodium hydroxide, water and catalyst into the autoclave, pass hydrogen into the autoclave, adjust to the appropriate pressure and temperature, and keep it until the end of the reaction. The feed liquid in the autoclave is taken out, the catalyst is removed by filtration, and the hydrogenation feed liquid is obtained. Use hydrochloric acid to adjust the pH value of the hydrogenation feed solution to about 2.5, and 1,3-cyclohexanedione crystals are precipitated, filtered, and dried to obtain 1,3-cyclohexanedione as a solid product.
[0059]The gas chromatography analysis process is as follows:
[0060] (1) Gas chromatography analysis conditions and parameters
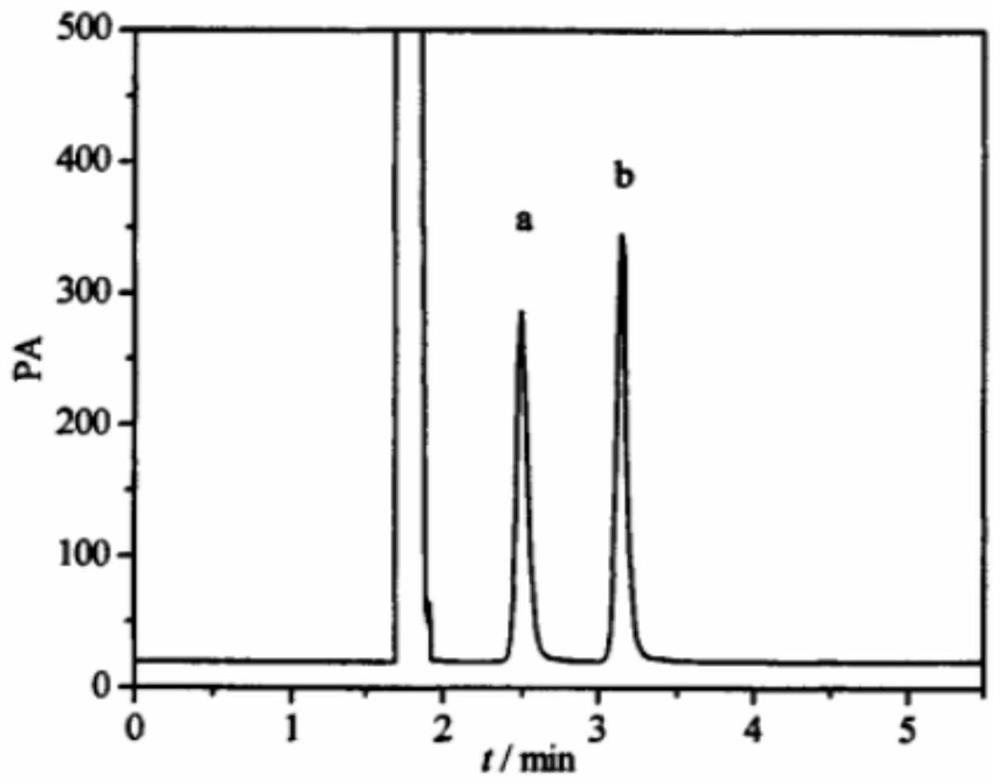
[0061] The gas chromatograph was Shimadzu GC-2010Pro, the gas chromatographic column was Agilent DB-624 (0.25mm×30.0m, 1.4μm), and nitrogen was used as the carrier gas. The injection volume was 1.0 μL, the gasification chamber temp...
Embodiment 2
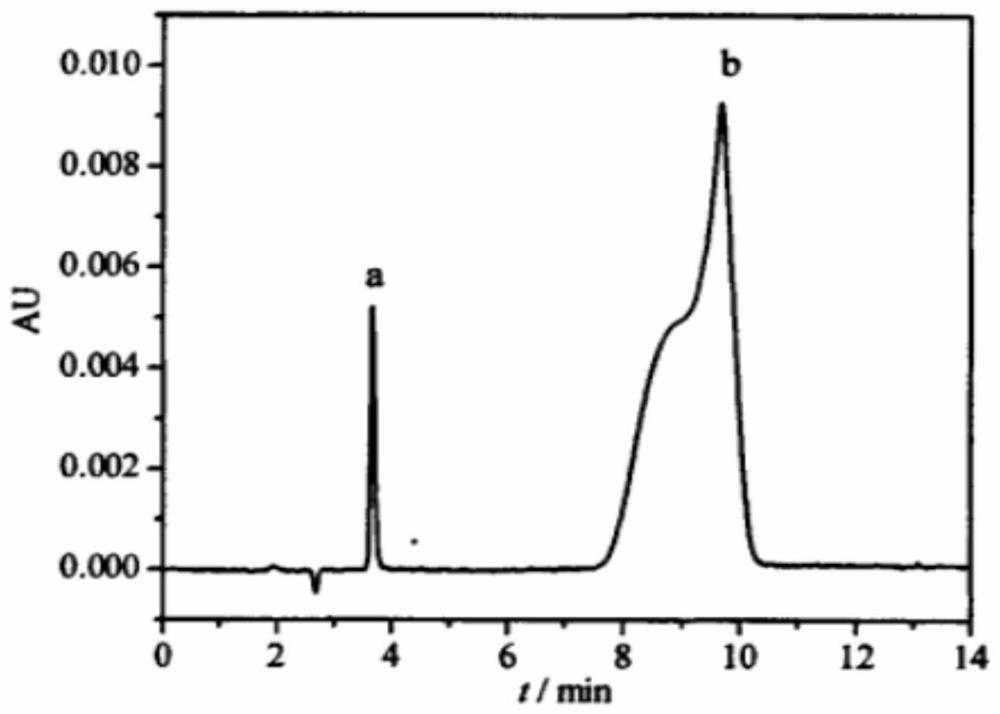
[0083] Compared with Example 1, the reverse-phase high performance liquid chromatography analysis condition part is adjusted as follows: the mobile phase is changed to V (acetonitrile: V (0.1% phosphoric acid aqueous solution)=20:80, and other conditions remain unchanged. Obtained The liquid chromatogram is as Figure 8 shown. It can be seen that under this condition, 1,3-cyclohexanedione and resorcinol are still well separated, and the peak shape still meets the requirements of quantitative analysis.
Embodiment 3
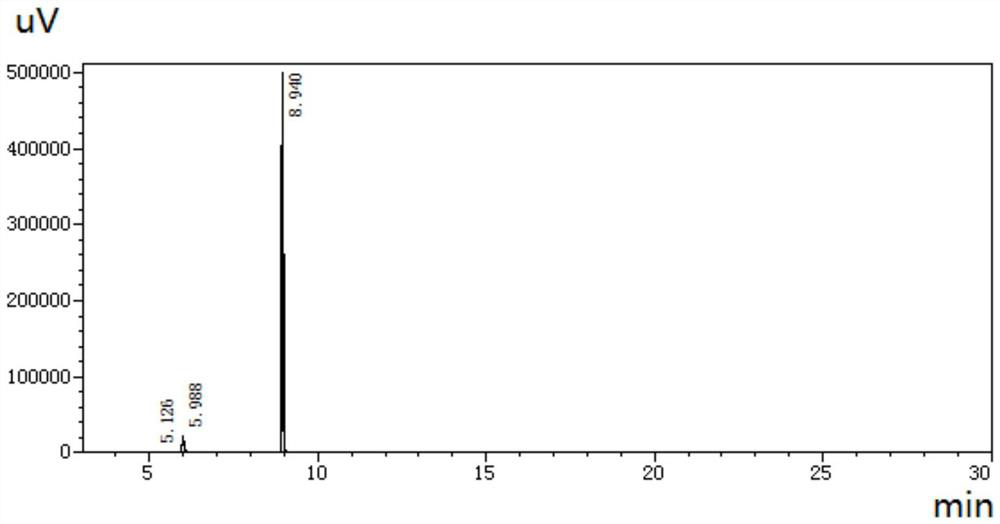
[0085] Compared with Example 1, the pH value of the mobile phase is adjusted to 4, and other conditions remain unchanged, and the liquid chromatogram obtained is as follows Figure 9 shown. Under this condition, the chromatographic peak shape of 1,3-cyclohexanedione is acceptable.
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com