Pharmaceutical composition as well as preparation method and application thereof
A composition and drug technology, applied in the directions of drug combination, drug delivery, pharmaceutical formulation, etc., can solve the problems of inability to fully exert sustained release, affect drug efficacy, and short sustained release time, and achieve the goal of facilitating intra-articular injection. drug, delaying the rate of biodegradation, and maintaining the balance of bone homeostasis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] The second aspect of the present invention provides a preparation method of a pharmaceutical composition, comprising: dissolving the release-controlling polymer in water, stirring to form a gel, and then adding interleukin-10.
[0032] In one or more embodiments of the present invention, the mass fraction of hyaluronic acid is 0.1-5%, preferably 2%; the concentration of interleukin-10 is 0.1-200 μg / mL, preferably 100 μg / mL.
[0033] The stirring temperature is 4-20° C., and the stirring time is 10-15 hours.
[0034] The third aspect of the present invention provides the application of a pharmaceutical composition in the preparation of medicines for treating IL-10-mediated diseases.
[0035] In one or more embodiments of the present invention, the IL-10-mediated disease is joint inflammatory disease.
[0036] In one or more embodiments of the present invention, the joint inflammatory disease is selected from rheumatoid arthritis, osteoarthritis and other inflammatory di...
Embodiment 1
[0040] Preparation and characterization of interleukin-10 combined with hyaluronic acid hydrogel system
[0041] (1) An appropriate amount of hyaluronic acid was dissolved in distilled water, the mass fraction of hyaluronic acid was 2%, and stirred at a low temperature of 10° C. for 12 hours to obtain a modified hyaluronic acid hydrogel.
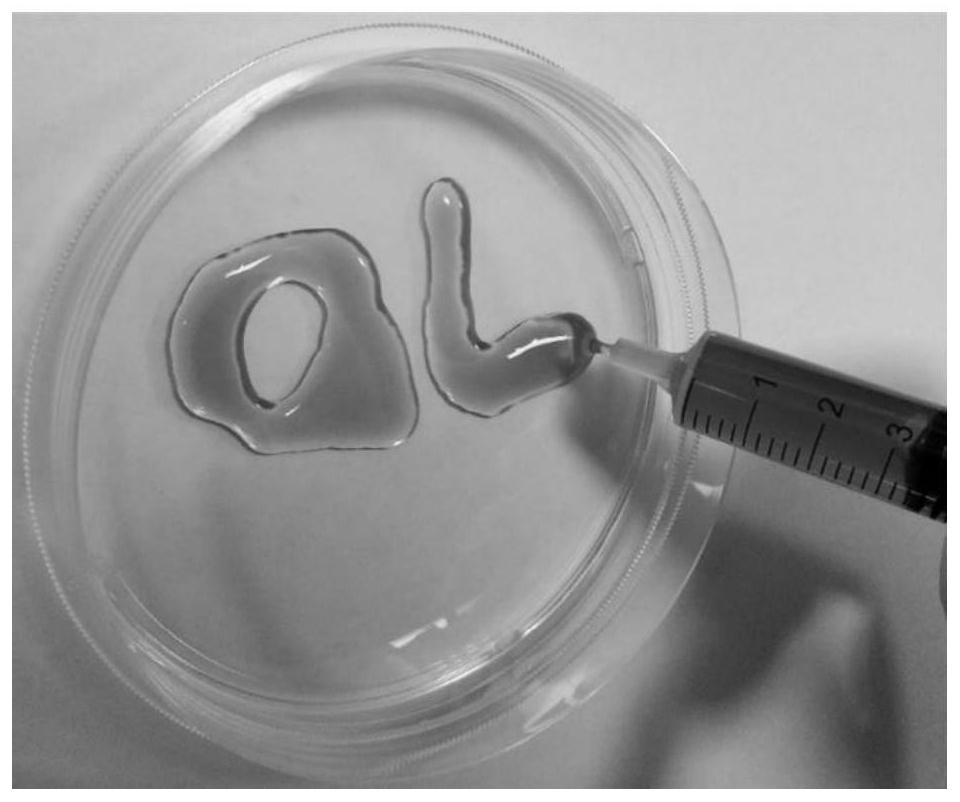
[0042] (2) Add interleukin-10 to the above-mentioned hyaluronic acid hydrogel and mix thoroughly at 250 rpm for 40 minutes, wherein the concentration of interleukin-10 is 100 μg / mL to obtain a hyaluronic acid hydrogel system loaded with interleukin-10. Prepared products such as figure 1 shown.
Embodiment 2
[0044] Application of interleukin-10 combined with hyaluronic acid hydrogel system in the treatment of osteoarthritis
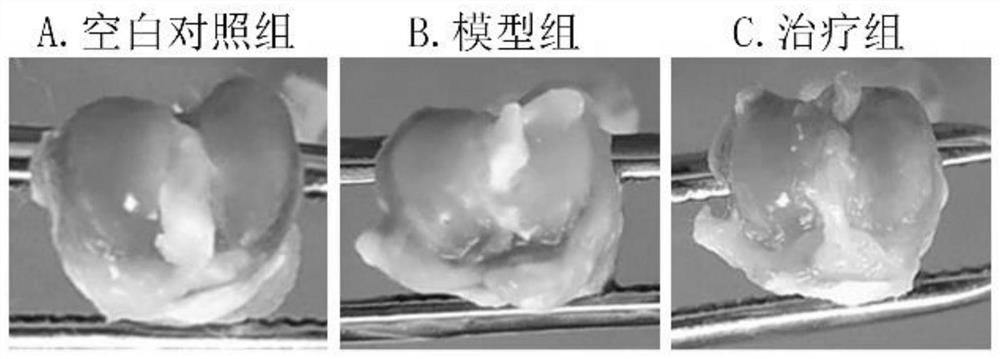
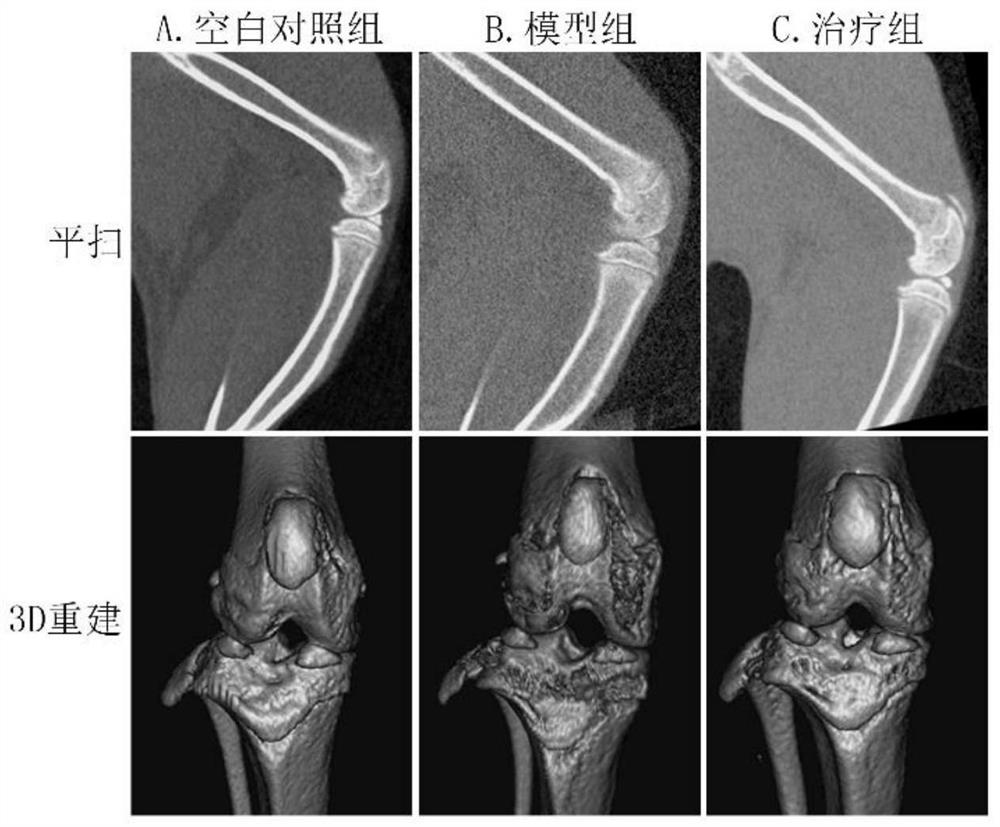
[0045] (1) Grouping of experimental animals: 15 rats were randomly divided into 5 as blank control group, and the remaining 10 rats were intra-articularly injected with sodium iodoacetate to construct an osteoarthritis model. Among the 10 model mice, 5 were randomly selected as the drug treatment group for intra-articular drug administration.
[0046] (2) Construction of animal model: 1% pentobarbital sodium intraperitoneal injection (40mg / kg) for anesthesia, skin preparation at the knee joint of rats in a sterile environment, iodophor disinfection, and sodium iodoacetate drawn with a micro-syringe The solution was injected into the knee joint cavity, and the injection dose for each rat was 3 mg (50 μL). The blank control group received no other treatment.
[0047] (3) Administration method:
[0048] The first group (blank control group): normal feeding. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com