A kind of divalent ionizable lipid compound, composition and application thereof
A compound and composition technology, applied in the field of bivalent ionizable lipid compounds, to achieve the effects of promoting development, good delivery efficiency, and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0159] Synthesis of Mon-01:
[0160] The synthetic route of Mon-01 is:
[0161] .
[0162] Step 1: Synthesis of a1:
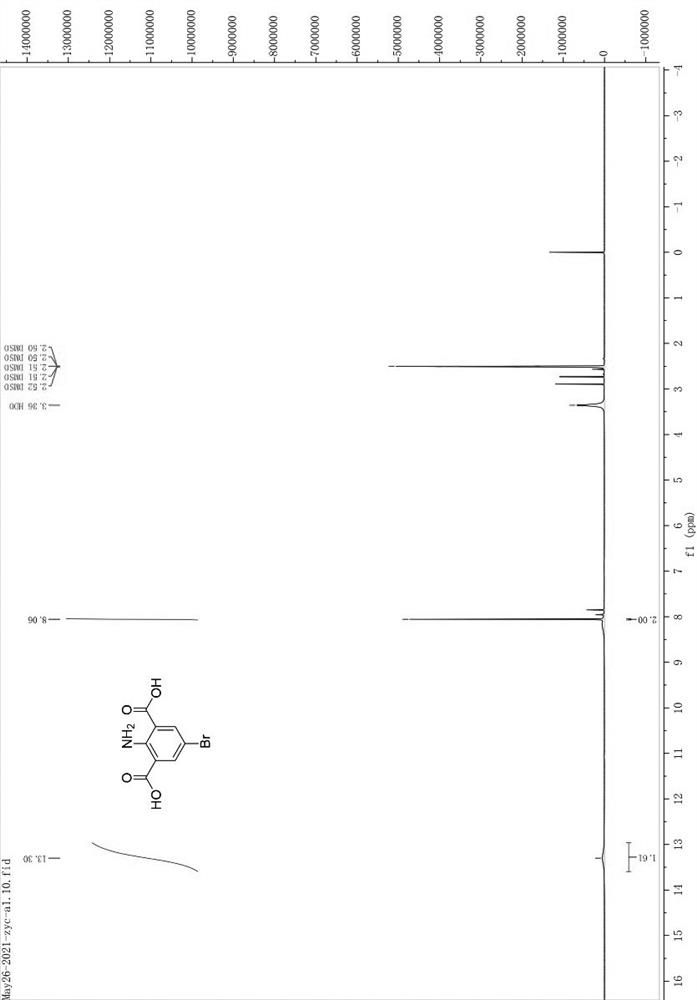
[0163] In a 250mL flask, weigh a0 (10.00g) and NBS (1.1eq) and dissolve them in 100mL of DMF, raise the temperature to 100°C, and react for 40min; spot the plate to monitor the progress of the reaction, and perform post-treatment after the reaction is complete; cool to room temperature, and react The liquid was poured into 300mL of ice water, stirred, filtered with suction, washed with water, dried, and dried to give a bright yellow solid (13.4g, 96.7% yield). The hydrogen spectrum of the compound is attached figure 1 . 1 HNMR (400 MHz, DMSO- d 6 ) δ 13.30 (s, 2H), 8.06 (s, 2H).
[0164] Step 2: Synthesis of a2:
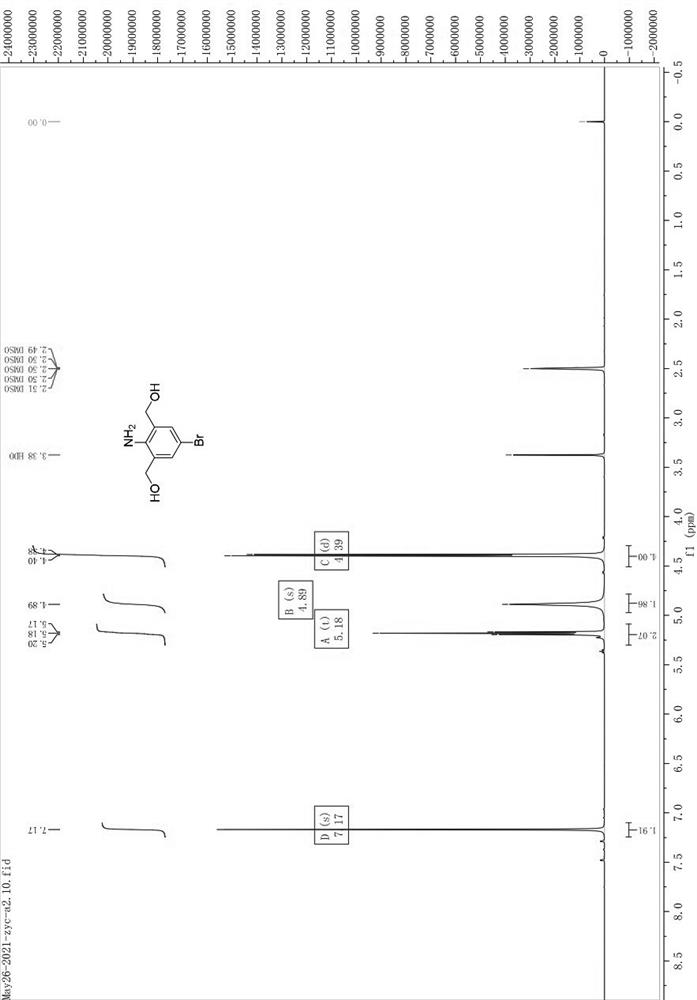
[0165] Weigh a1 (10.00g) and dissolve it in 100mL of THF, stir in ice bath for 15min; under ice bath, add BH dropwise 3 / THF (1.0M, 6eq), return to room temperature after the dropwise addition, and stir overnight; spot the plate to monitor ...
Embodiment 2
[0185] Synthesis of hydrophobic aliphatic chain tail T-4-7:
[0186] The synthetic route of T-4-7:
[0187]
[0188] Step 1: Synthesis of T-2-2:
[0189] In a 250mL single-necked bottle, weigh T-2-1 (2.22g, 10mmol) and dissolve it in 30mL of anhydrous DMSO, 10 o C and stirred for 5min, added TosMIC (195.03, 0.98g, 5mmol), stirred for 5min, added NaH (0.48g, 12mmol) in batches, and finally added TBAI (369.37, 0.37g, 1mmol), slowly raised to room temperature and stirred overnight, and plated Monitor the progress of the reaction (PE: EA = 49: 1), the reaction is complete, cool the reaction solution in an ice-water bath, slowly add 80 mL of ice water to quench, extract with DCM (60 mL*3), combine the organic phases, wash with 80 mL of water, Wash with saturated sodium bicarbonate (80mL*2), wash with brine, dry, and concentrate to obtain the crude product T-2-2, which is directly carried out to the next reaction.
[0190] Step 2: Synthesis of T-2-3:
[0191] In a 100mL singl...
Embodiment 3
[0201] Synthesis of Divalent Cationic Lipid Compound 010101:
[0202] 010101 synthetic route:
[0203]
[0204] Step 1: Synthesis of 0101:
[0205] Weigh cationic group compound (450mg, 6.0eq) and DIEA (340mg, 6.0eq) dissolved in 35mL of DCM, stir at room temperature; weigh Mon-01 (300mg, 0.43mmol) dissolved in 15mL of DCM to add to the reaction solution, point The progress of the reaction was detected on a plate, and the reaction was completed after treatment; washed with water, washed with brine, dried, concentrated, and mixed with a column for purification (160 mg, 53.3% yield). The hydrogen spectrum of the compound is attached Figure 15 . 1 H NMR (400 MHz, Chloroform- d ) δ 8.17 (s, 1H), 8.11 (d, J = 8.1 Hz,1H), 7.74 (s, 2H), 7.51 (s, 3H), 6.37 (s, 2H), 5.60 (s, 2H), 5.05 (s, 4H),3.50 (s, 8H), 3.20 (s, 4H), 3.10 (s, 1H), 2.62 – 2.47 (m, 12H).
[0206] Step 2: Synthesis of 010101:
[0207] In a 10mL single-necked bottle, weigh 0101 (20mg, 0.028mmol) and T-4-7 (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com