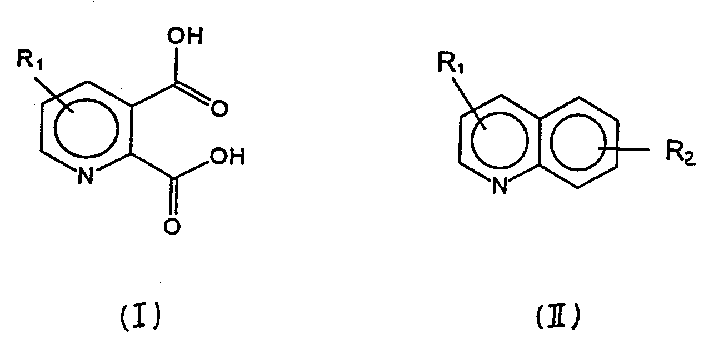
Process for preparation of 2,3-pyridine dicarboxylic acid
A technology of dipicolinic acid and sulfuric acid, applied in directions such as organic chemistry, can solve problems such as unsatisfactory purity of dipicolinic acid, and achieve the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024]140 g (1.08 mol) of quinoline 97.5%, Sumitomo containing 1.5% isoquinoline was dissolved in 1770 g of distilled water and 212.6 g of 98% sulfuric acid (2.16 mol) to obtain an approximately 7% solution with a pH of 0.3 . An oxygen stream containing ozone was passed through the solution in a circulating ozonizer at 6°C. The duration of the ozonolysis reaction was 8 hours and 40 minutes and 118 grams (2.46 moles) of ozone were consumed. 3 drops of antifoam (Antifoam SRE, Wacker) diluted in 50 ml of water were added as needed for a total of 17 ml of antifoam solution.
[0025] When the ozonolysis was complete, 2094 grams of peroxide solution were obtained, and 216 grams (1.86 moles) of hydrogen peroxide (30% strength) were added to the solution under stirring in a reaction vessel precooled to 20°C. The temperature rose to 24°C during the addition. The reaction mixture was stirred overnight, then 305 g of 40% NaOH was added at a temperature of 20-30°C to adjust the pH from...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com