Composition for preventing and/or treating osteoporosis
A technology for osteoporosis and composition, which is applied in the fields of health care products or food and medicine to achieve the effect of preventing various diseases and improving the nutritional level of the human body
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1: Ecdysterone Composition (βEcd-combo) Increases Osteogenesis Test
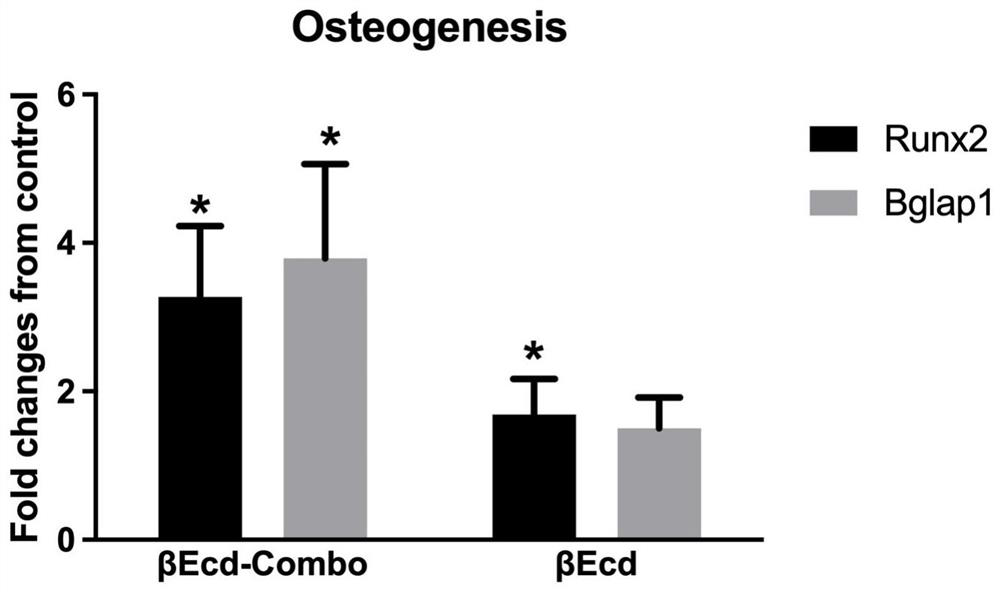
[0036] 1.1 Use 2 mL each of βEcd and βEcd-combo in osteogenic medium (ThemoFisher Scientific) at 10 -7 Mol cultured bone marrow stromal cells for 28 days. Osteogenic gene expression (RUNX2 and Bgalp) was measured on day 7 and eligibility for bone mineralization nodule formation was measured on day 21. The result is as figure 1 shown. This result indicated that βEcd-combo was more able to promote osteogenesis in vitro by the fold change compared with βEcd control treatment. exist figure 1 In the histogram of osteogenic gene expression, the results given are that βEcd-combo is more effective than βEcd in promoting osteogenic RUNX2 and Bgalp gene expression.
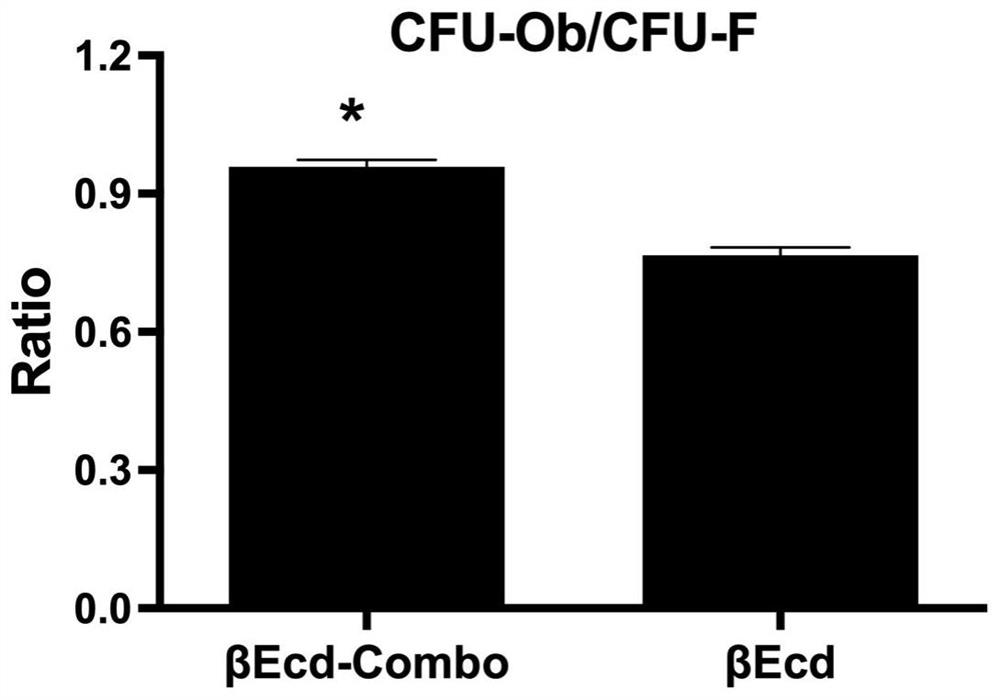
[0037] 1.2 Mix bone marrow stromal cells with 2 mL each of βEcd and βEcd-combo in 10 -7 Mol was cultured for 28 days and then stained for crystal violet representing total colony formation. The same cell culture wells were then used ...
Embodiment 2
[0038] Example 2: Effect test of ecdysterone composition (βEcd-combo) on osteoporosis caused by estrogen deficiency
[0039]2.1 Two-month-old female mice were randomly divided into 3 experimental groups, with 8 animals in each group. Group 1, normal mice, ie control group; Group 2, ovariectomized group (OVX); treatment started immediately after OVX. Group 3, ovariectomized + βEcd-combo (OVX + βEcd-combo, oral gavage βEcd-combo, the dosage is equivalent to 0.5mg / kg Achyranthes bidentata, 0.25mg / kg quinoa, 0.25mg / kg white mulberry leaf , administered 5 times a week, that is, 5 days of administration per week and 2 days of withdrawal). Mice were euthanized 4 weeks after dosing.
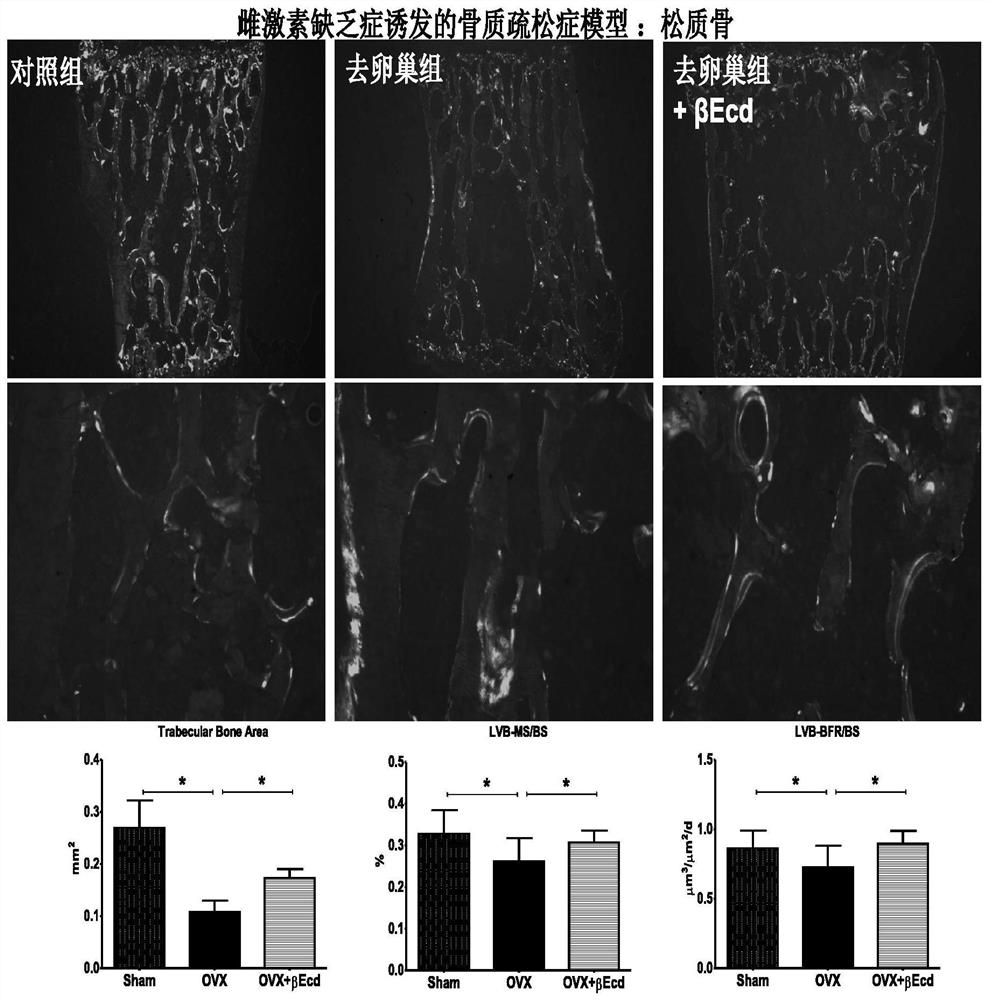
[0040] 2.1.1 Cancellous bone test results see image 3 .
[0041] The results showed that bone mass and bone formation (total amount of white fluorescently labeled bone surface) were reduced after estrogen deficiency (ovariectomy). In this primary osteoporosis model, administration of the ecdysteron...
Embodiment 3
[0044] Embodiment 3: The impact test of ecdysterone composition (βEcd-combo) on the osteoporosis induced by glucocorticoid
[0045] 3.1 Two-month-old male Swiss-Webster mice were randomly divided into 4 experimental groups, with 8 mice in each group. The implantation method of sustained-release pellets of prednisolone (GC) (Innovative Research Corporation of America, Sarasota, Florida) was as follows: Group 1, control group, implanted placebo pellets (PL); group, implanted PL pellets + βEcd (PL+βEcd-combo 0.5mg / kg, administered 5 times a week); group 3, implanted prednisolone 5mg / 60-day sustained-release pellets, equivalent to 2.8 mg / kg / d (GC); the 4th group, implanted prednisolone 5mg / 60 day sustained-release pellets+βEcd (GC+βEcd-combo, the dosage of ecdysterone composition is equivalent to 0.5mg / kg Achyranthes bidentata, 0.25mg / kg quinoa, 0.25mg / kg white mulberry leaves)), and the ecdysterone composition was administered orally, administered 5 times a week). Wherein, the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com