Construction method and application of GLUD1 mutant gene knock-in mouse animal model
A technology of mutated genes and animal models, applied in the field of biomedicine, can solve problems such as undiscovered congenital hyperinsulinemia, and achieve good clinical application prospects, wide application value, and great social benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
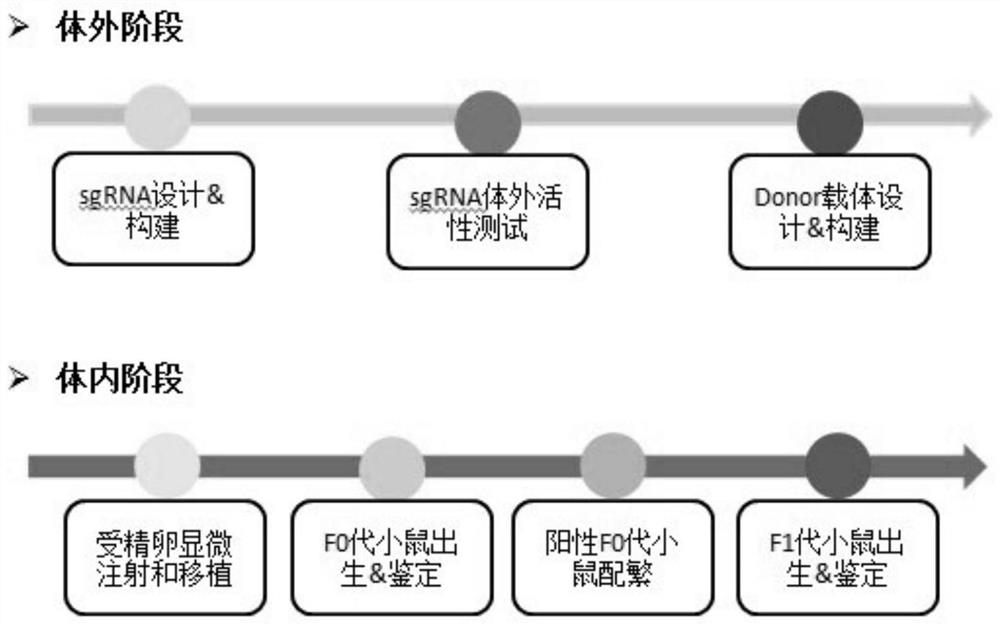
[0052] Example 1 Cas9 / gRNA target design and in vitro Cas9 enzyme cleavage activity detection
[0053] (1) Experimental animals
[0054] 6-week-old healthy C57BL / 6 mice (weight 18±2g), 100 male and 100 each, provided by Shanghai Southern Model Biotechnology Co., Ltd. After two weeks of isolation and rearing, they can reach the age of 8 weeks required by the experiment ; 60 healthy B6D2F1 mice aged 6-8 weeks, half male and half female, weighing 24±2g, were purchased from Shanghai Southern Model Biotechnology Co., Ltd., and the mice were fed in an SPF-grade environment in strict accordance with the animal feeding environment The management regulations ensure that the daily temperature is 22°C, the humidity is 40% to 60%, and the light time and dark time in the animal room are half and half. All related operations and handling of animal experiments strictly follow the relevant management regulations on experimental animals.
[0055] (2) Experimental reagents
[0056] Cas9 Nuc...
Embodiment 2
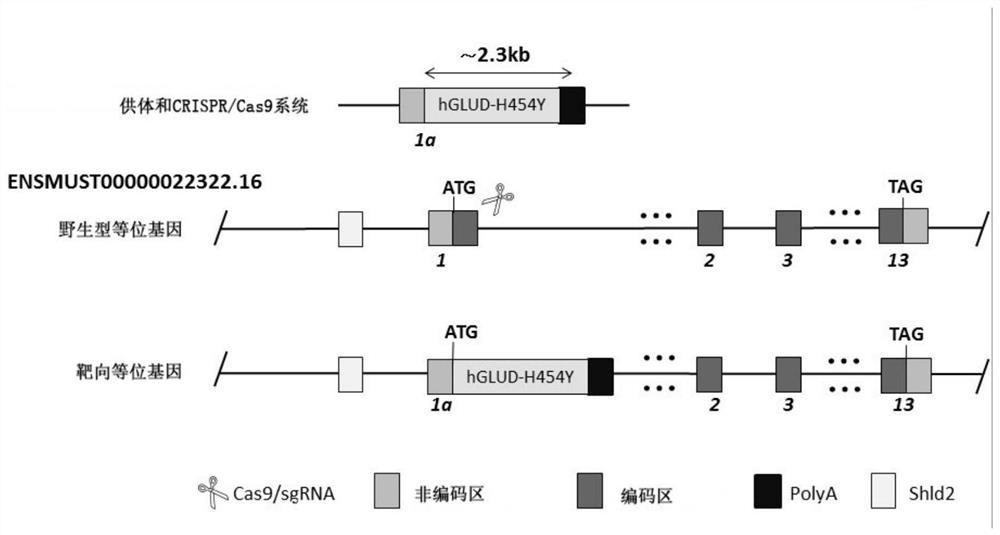
[0069] Embodiment 2 Construction of Donor vector
[0070] The homologous recombination vector (Donor vector) was constructed by the method of In-Fusing cloning. Wherein, the base sequence information of the constructed Donor vector is shown in SEQ ID NO:2.
[0071] SEQ ID NO: 2
[0072]GATTTGCCCCGCGCCTCTTCGGCTTCGGCGCGGCGTCACTCTCCGGCCGGCCGCGCAGCGGATCCGCACACCGTTCCGGCCCAAGGCGGAAAGGAGGGGCCTGGTGACTCATGCGGCCGGGGCTGCTGGCCAGCCAGCGAGGCCTCCCTGCAGTCCGCTTCCACCTCCCAGGCCGCCGCGCGTGCACACGGGACCGACCTTCCGGGCCGCGGCCGCGTCCGACCGCTCTCAGGAGCCCAGGCCTCCGGGCCGCAGCCCGCGCGCGTCCACGCTAGCCCCGCCCCCGCCCGCGAGCATGCGCACCAAGCCCATCTGCAAAGGCGCGCCCCCCGCCGCCGCAGCCGCACGCGCCGCCGCCTGGAGGCCGGGCAAGGCCGCGGCGGAGGCGAGGCCCAGCGCCCTGGGCGGCGCGCGGCCGCCGAAGTCCGTCCTCCCGGTGGGGCGACAAGCGGCGCAGGGGAGGGGACAGCCAGACAAGCAGGAAGCCGCGGCTTAAAAGGGCAGCTCGCGCCCAGCCCTTCCTCCCCGCGGTCCAGGCCTGCGAGCTCCGGTCTTTACAGCTCCCGCCGCACTCGCCTCAGCCCGCCGCCGCCGCCATGTACCGCTACCTGGGCGAAGCGCTGTTGCTGTCCCGGGCCGGGCCCGCTGCCCTGGGCTCGGCGTCCGCCGACTCGGCCGCGTTGCTGGGCTGGGCCCGGGGAC...
Embodiment 3
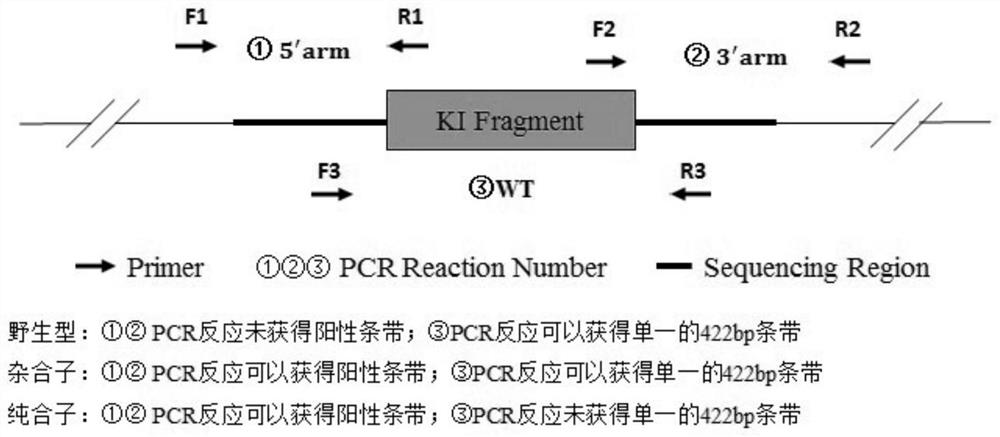
[0073] Example 3 Microinjection
[0074] (1) Component preparation: The microinjection components were prepared according to Table 3, and the prepared components were subjected to high-speed centrifugation at 4°C, and 15 μL was taken out after 10 min for subsequent microinjection experiments.
[0075] Table 3 Concentration and consumption of each component of microinjection
[0076] components concentration Dosage Cas9 mRNA 200ng / μl 5μl Donor vector 200ng / μl 5μl sgRNA 200ng / μl 5μl Add RNase-free H 2 O to
40μl 25μl
[0077] (2) Ovulation treatment of embryo donor mice
[0078] Prepare 8-week-old and healthy C57BL / 6J female mice, hormones pregnant horse serum gonadotropin (PMSG) and human chorionic gonadotropin (HCG). PMSG was injected at 14:30 in the afternoon, and the concentration of PMSG injected into each female mouse was 10 units (10 IU / mL), and the dose was 0.2 ml. After 46-48 hours, HCG was injected again. Th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com