Application of epigallocatechin gallate in preparation of medicine for preventing or treating systemic jellyfish toxin poisoning
A technology of epigallocatechin and gallate, applied in the field of biomedicine, can solve problems such as prevention or treatment of systemic poisoning of jellyfish toxin, achieve good clinical application promotion value, improve blood pressure reduction, and improve survival rate Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1: the groping of mouse intravenous injection jellyfish toxin (NnV) lethal dose
[0038] 1. Experimental method
[0039] The ICR mice were randomly divided into 5 groups (n=3), and different doses of NnV (0.42mg / kg, 0.5mg / kg, 0.6mg / kg, 0.72mg / kg, 0.86mg / kg, the solvent was physiological saline, dilute to 0.1ml), and observe the effects of different doses of NnV on the survival of mice.
[0040] 2. Experimental results
[0041] When the toxin dose was ≥0.6mg / kg, the mice died within 2 hours. When the toxin dose was 0.5 mg / kg, the survival time of the mice was prolonged, but all died within 1 day. When the toxin dose was 0.42mg / kg, some mice could survive for more than 1 week.
Embodiment 2
[0042] Embodiment 2: Survival analysis of NnV poisoned mice
[0043] 1. Experimental method
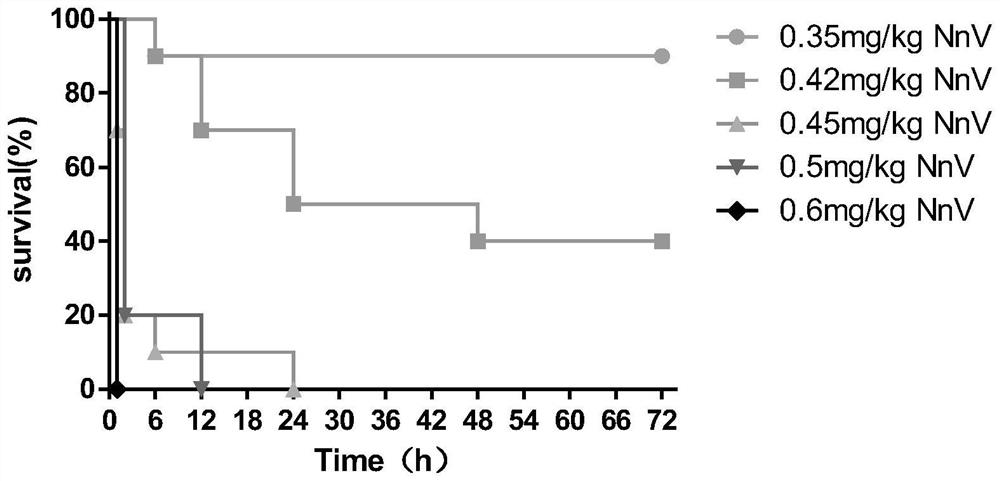
[0044] According to the results of Example 1, the dose range of NnV was re-determined, and the number of mice in each group was expanded. The ICR mice were randomly divided into 5 groups (n=10), and different doses of NnV (0.35mg / kg, 0.42mg / kg, 0.45mg / kg, 0.5mg / kg, 0.6mg / kg, the solvent was physiological saline, dilute to 0.1ml), and observe the effects of different doses of NnV on the survival of mice.
[0045] 2. Experimental results
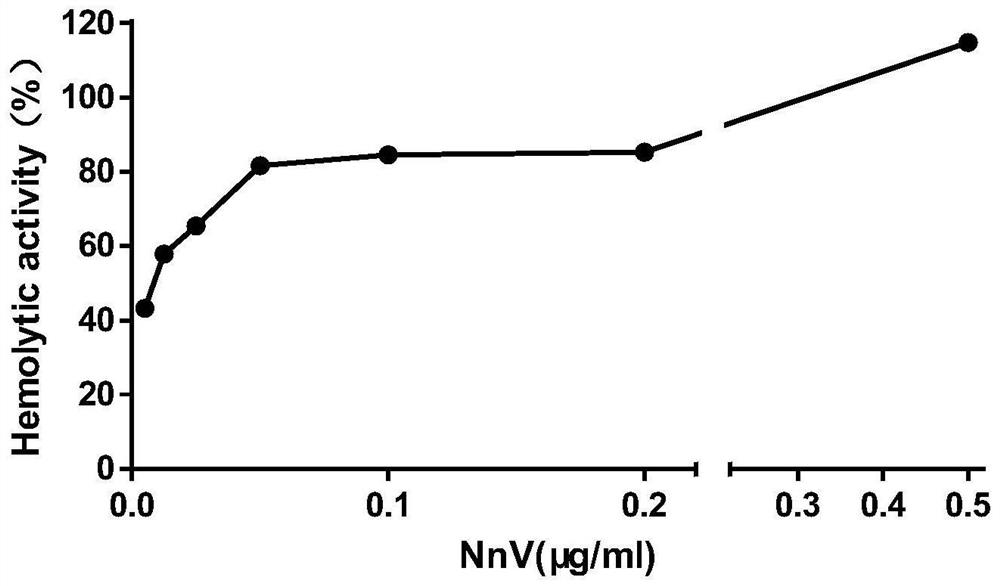
[0046] Such as figure 1 As shown, when the toxin dose was less than or equal to 0.42mg / kg, the mice showed an excitatory response in the early stage, and gradually appeared lethargic, unresponsive, and curled up after 10 minutes, and some mice died within 3 days. When the toxin dose was 0.45mg / kg or 0.5mg / kg, the mice had shortness of breath, increased heartbeat, fatigue, lethargy, curling up, convulsions and death in the early stage. However, wh...
Embodiment 3
[0047] Embodiment 3: Exploration of the effective dose range of EGCG antagonizing lethal dose NnV
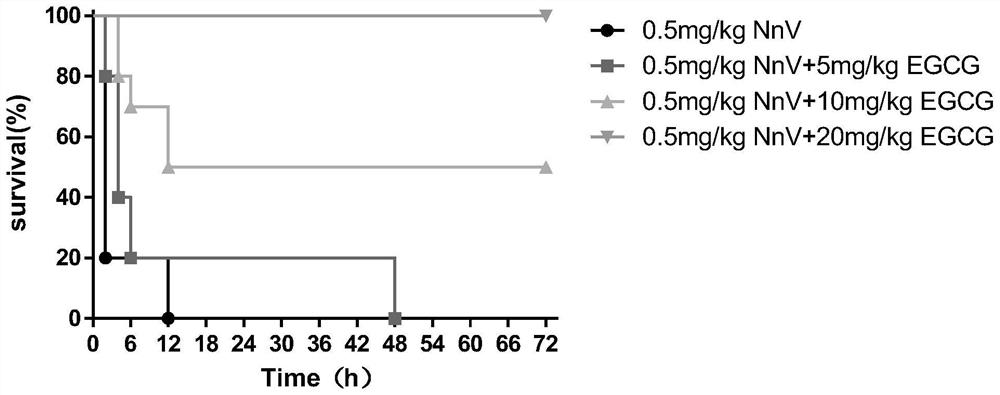
[0048] 1. Experimental method
[0049] EGCG was made into a 10mg / ml solvent with physiological saline. ICR mice were randomly divided into 5 groups (n = 2) and different doses of EGCG (100mg / kg, 80mg / kg, 40mg / kg, 20mg / kg, 10mg / kg) were mixed with 0.5mg / kg NnV, and then fixed with normal saline. The volume was reduced to 0.2ml, and injected into the tail vein, and the reaction and survival time of the mice were observed.
[0050] 2. Experimental results
[0051] Except one mouse died in the 10mg / kg EGCG group, all the other mice survived for 7 days. The mice in the dose group above 20mg / kg showed mild excitement in the early stage, and returned to normal after about 2 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com