A monoclonal antibody yg11-2 against Staphylococcus aureus enterotoxin b and its application
A golden yellow, enterotoxin technology, applied in the direction of antibodies, applications, antibacterial drugs, etc., can solve the problem of SEB poisoning vaccines and drugs, etc., and achieve the effect of high affinity and improving survival rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
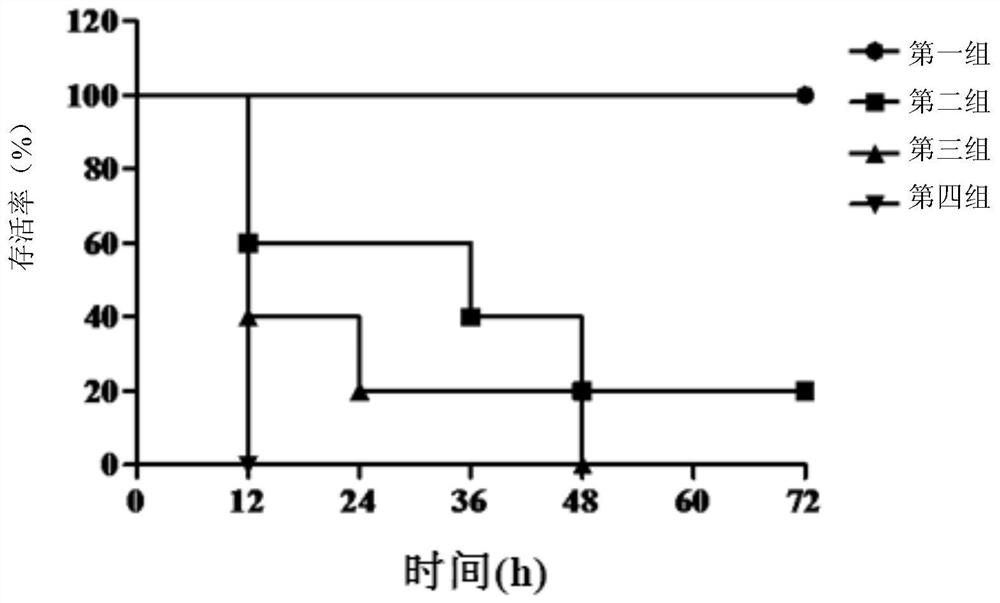
Examples
Embodiment 1
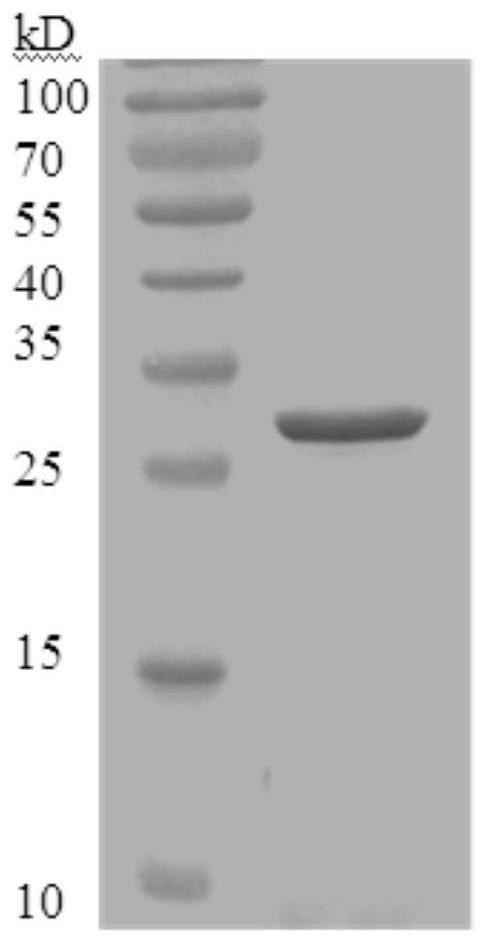
[0039] Embodiment 1, the preparation of His-SEB protein and its functional research
[0040] 1. Preparation of His-SEB protein
[0041] 1. Replace the small fragment between the NdeI and XhoI restriction sites of the vector pET28a(+) with the DNA molecule shown in sequence 6 of the sequence listing to obtain the recombinant plasmid pET28a-SEB. Sequencing verified.
[0042] In the recombinant plasmid pET28a-SEB, the DNA molecule shown in sequence 6 in the sequence table is fused with the DNA molecule encoding His tag on the vector backbone to form a fusion gene to express the fusion protein.
[0043] The fusion gene is composed of the following two segments sequentially from upstream to downstream: the DNA molecule shown in sequence 8 in the sequence listing, and the DNA molecule shown in sequence 6 in the sequence listing.
[0044] The fusion protein consists of the following two segments sequentially from the N-terminal to the C-terminal: the segment shown in sequence 7 of ...
Embodiment 2
[0073] Embodiment 2, phage antibody library screening anti-SEB protein monoclonal antibody
[0074] 1. Biopanning of anti-SEB monoclonal antibodies
[0075] (1) SEB group
[0076] 1. The first round of affinity panning
[0077] (1) Coat the immunotube with 500 μL of His-SEB protein solution (adjust the protein concentration with PBS buffer) with a protein concentration of 10 μg / mL, and overnight at 4°C.
[0078] (2) Take the immunotube, add PBST buffer solution containing 5g / 100mL skimmed milk powder, and block at room temperature for 1 hour.
[0079] (3) Take the phage antibody library, add PBST buffer containing 5g / 100mL skimmed milk powder, and block at room temperature for 1 hour.
[0080] (4) Take the immune tube that completed step (2), first add sterile PBS buffer to wash 3 times, and then add the phage antibody library that completed step (3) (the input amount of phage is about 1.2×10 12 ), let stand at room temperature for 1h.
[0081] (5) After completing step (...
Embodiment 3
[0101] Example 3, Functional Identification of YG11-2 Antibody
[0102] 1. Preparation of YG11-2 antibody
[0103] 1. Construction of recombinant plasmids
[0104]The small fragment between the HindIII and XhoI restriction sites in the vector pCDNA3.1(+) was replaced with the DNA molecule shown in Sequence 2 of the sequence listing to obtain the heavy chain expression vector. In sequence 2 of the sequence listing, nucleotides 1-584 constitute a promoter, nucleotides 681-2108 encode a full-length heavy chain, and nucleotides 2141-2196 constitute a terminator.
[0105] The small fragment between the HindIII and XhoI restriction sites in the vector pCDNA3.1(+) was replaced with the DNA molecule shown in Sequence 4 of the sequence listing to obtain the light chain expression vector. In sequence 4 of the sequence listing, nucleotides 1-584 constitute a promoter, nucleotides 681-1388 encode a full-length light chain, and nucleotides 1421-1476 constitute a terminator.
[0106] 2. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com