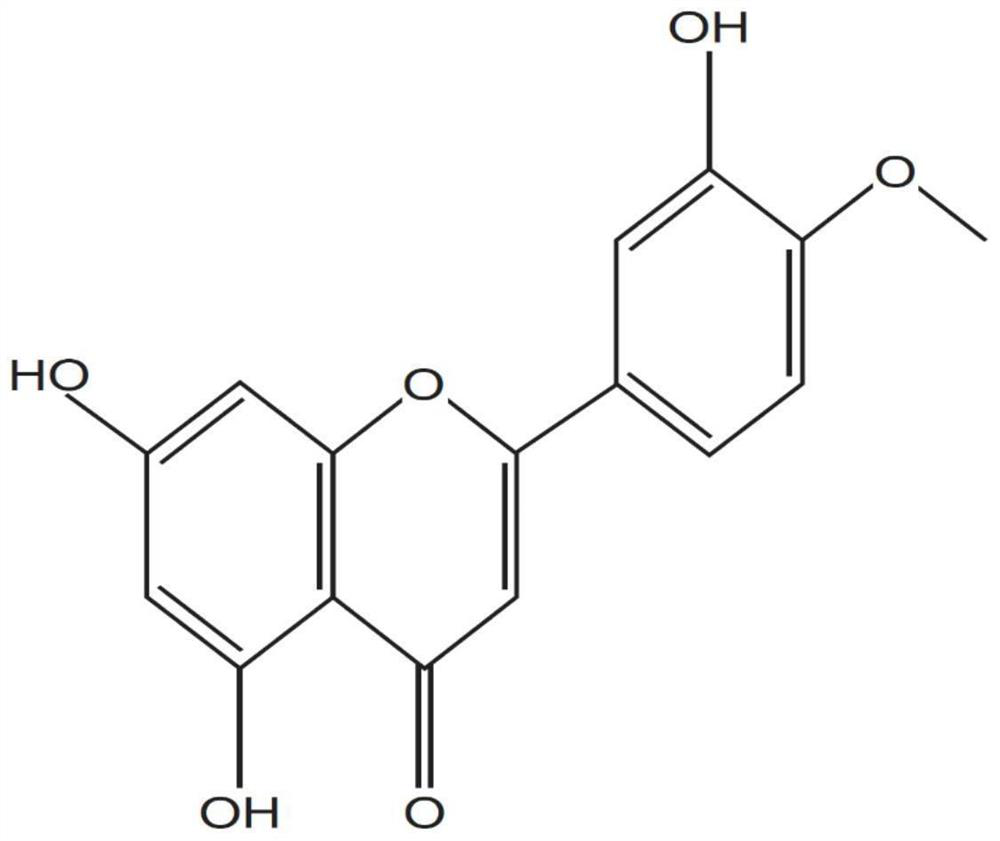
Application of diosmetin and method for establishing animal model of diosmetin
A technology of diosmin and animal models, applied to medical preparations containing active ingredients, drug combinations, pharmaceutical formulas, etc., can solve problems such as lupus erythematosus without diosmin, and achieve low potential side effects and a large safe concentration range , the effect of strong stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1, cell culture
[0045] THP1, raji, and jurkat cells were obtained from the American Type Culture Collection (ATCC). When recovering, take out the frozen cells from the liquid nitrogen tank, quickly place them in a 37°C water bath, and shake slightly to speed up the dissolution; add the frozen liquid to 9ml of preheated cell culture medium, mix them upside down; centrifuge at 20°C and 90G for 5 Minutes; remove the supernatant, add 1ml of cell culture medium to resuspend the cells, transfer the cells to a cell culture dish or flask, and indicate the cell type, generation, date and name. Culture in a 25cm cell culture flask containing 1640 (plus 10% calf serum) medium, and transfer to a 75cm cell culture flask when the number of cells increases enough. All cells were cultured in a 37°C incubator containing 5% CO2, subcultured and replaced every 3 days, and the number of cell generations used in the experiment was maintained within 20 passages.
Embodiment 2
[0046] Embodiment 2, real-time fluorescent quantitative PCR (Real-time PCR)
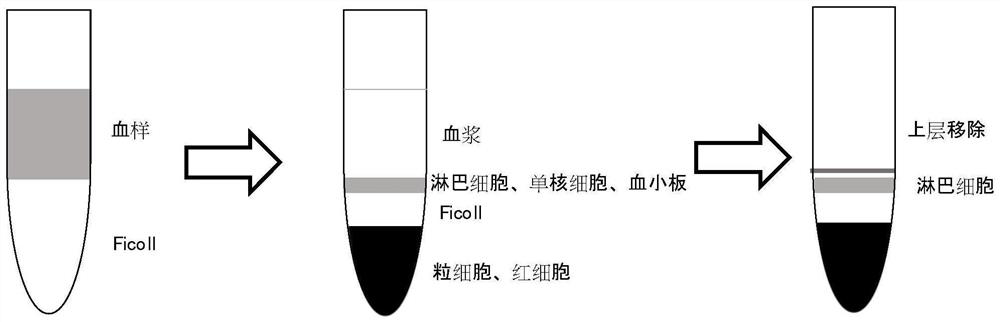
[0047] PBMC from healthy people were collected, and CD3+T cells, CD19+B cells, and CD14+monocytes were obtained by magnetic bead sorting. The experimental group was diosmin, and the control group was DMSO. Cells were cultured in 24 wells, and diosin and DMSO were added. The control was incubated for 30min, and then Type I IFN (1000u / ml, PBL company) was added to stimulate. After stimulation for 0, 2h, 4h and 6h, the supernatant was removed and extracted using phenol-chloroform method. Cells in each well were 0.5mL Trizol (Invitrogen company ) to collect RNA, add 100uL chloroform and mix well, let stand for 3 minutes, centrifuge at 12000g 4°C for 15 minutes, absorb the supernatant and add 300uL isopropanol, mix well, let stand for 10 minutes, centrifuge at 12000g 4°C for 10 minutes, remove the supernatant, Wash twice with 500uL of 75% ethanol, and centrifuge at 4°C for 5 minutes at 7500g each time. I...
Embodiment 3
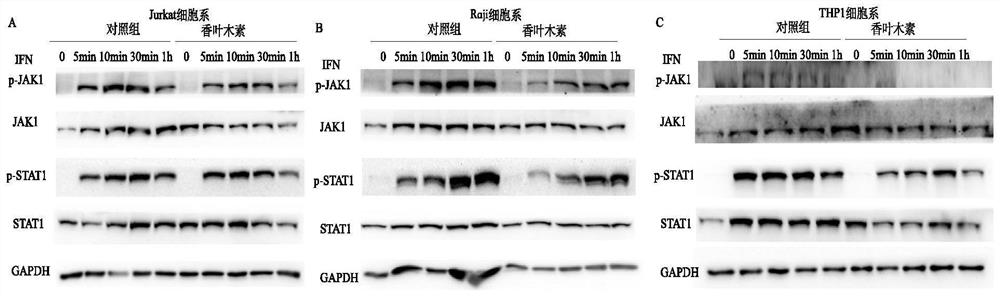
[0050] Embodiment 3, immunoblotting experiment (Western Blot)
[0051] THP1, raji, and jurkat cells were cultured in 6-well plates, diosmin and DMSO were added to incubate for 30min, and then Type I IFN (1000u / ml, PBL company) was added to stimulate for 0, 5min, 10min, 30min, 1h, and then removed The supernatant was extracted with the lysis method of RIPA Buffer (product of Thermo Company), and the obtained protein was diluted with 5×SDS-PAGE buffer solution (product of Beyotime Company), and denatured. The Bio-Rad vertical electrophoresis system was used for protein electrophoresis, and then the Bio-Rad wet transfer system and PVDF membrane were used for protein transfer, and then the PVDF membrane was blocked with BlockingBuffer (Thermo company product), the primary antibody was incubated overnight at 4°C, washed with PBST, and the secondary antibody Incubate at room temperature for 1 h, wash with PBST, and develop using Pierce ECL Western Blotting Substrate (product of Ther...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com