Fibrin gel containing adriamycin-entrapped platelet exosome and PD-L1 monoclonal antibody as well as preparation method and application of fibrin gel
A fibrin gel, PD-L1 technology, applied in the field of medicine, to achieve the effect of preventing tumor recurrence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
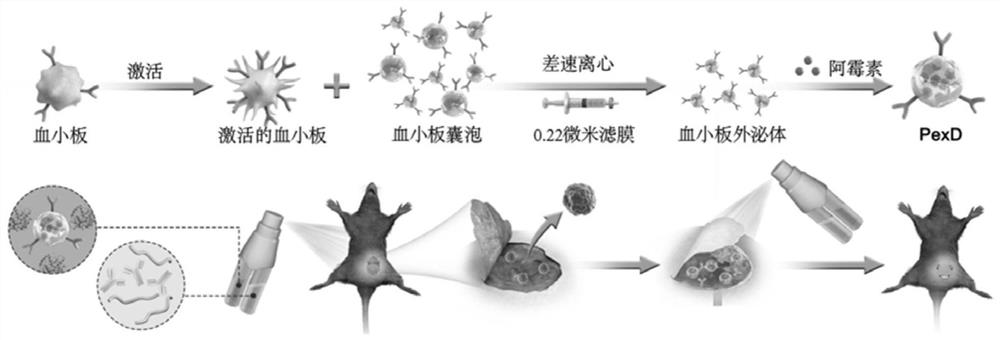
[0095] Preparation and characterization of platelet-encapsulated doxorubicin and bioreactive fibrin gels.
[0096] Synthesis schematic see figure 1 .
[0097] 1) Preparation of platelet exosomes.
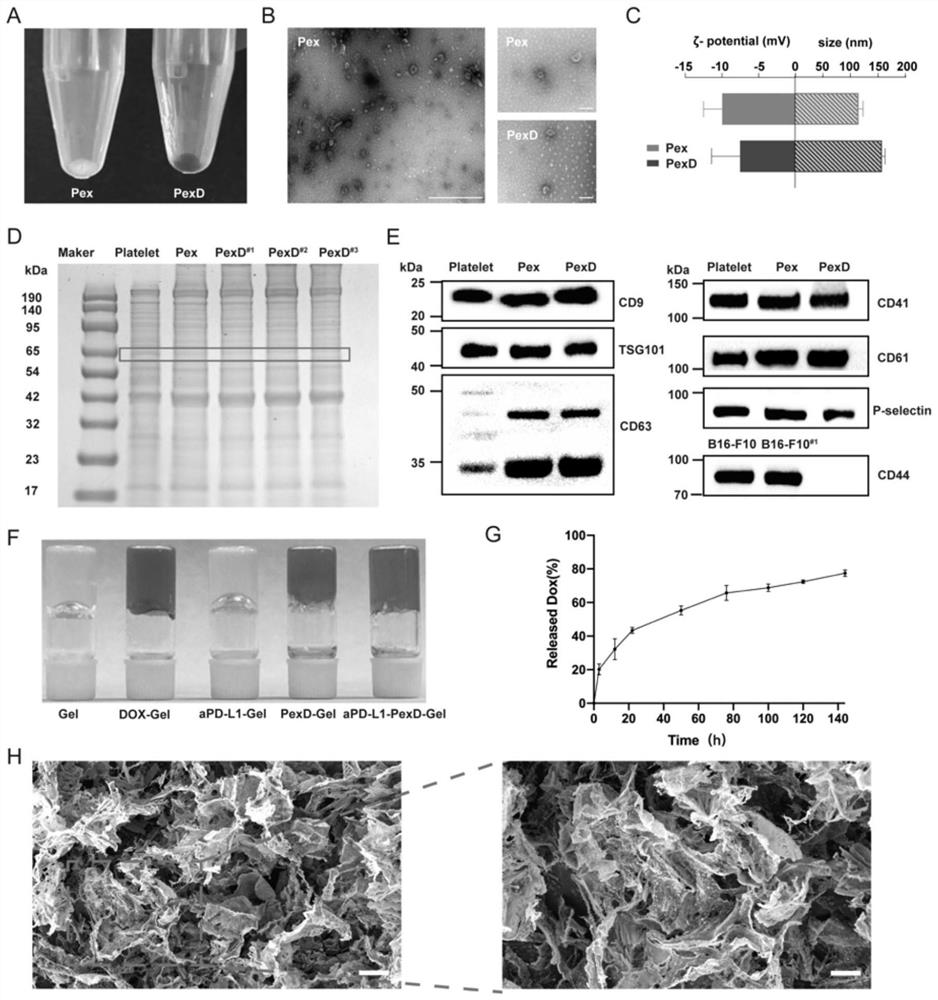
[0098] After platelet-rich plasma was anticoagulated with EDTA, red blood cells were removed by centrifugation at 100g for 20 minutes, the supernatant was added to ACD solution to prevent platelet activation, and platelet membrane was prepared by centrifugation at 800g for 20 minutes. Tyrode-HEPES buffer (1mM MgCl 2 , 2mM CaCl 2 and 3mM KCl 2 ) to dilute platelets to 250×10 6 platelets / mL, and bound Ca 2+ ionophore (10 mM, Sigma-Aldrich), incubated for 30 minutes, and then centrifuged at 800 g for 10 minutes. The collected supernatant was further ultracentrifuged, and the extracellular vesicles were ultracentrifuged at 100,000 g for 90 minutes to concentrate the particles. After resuspension, the extracellular vesicles were passed through a 220nm microporous membrane to obta...
Embodiment 2
[0105] Adhesion of platelet exosomes to tumor cells.
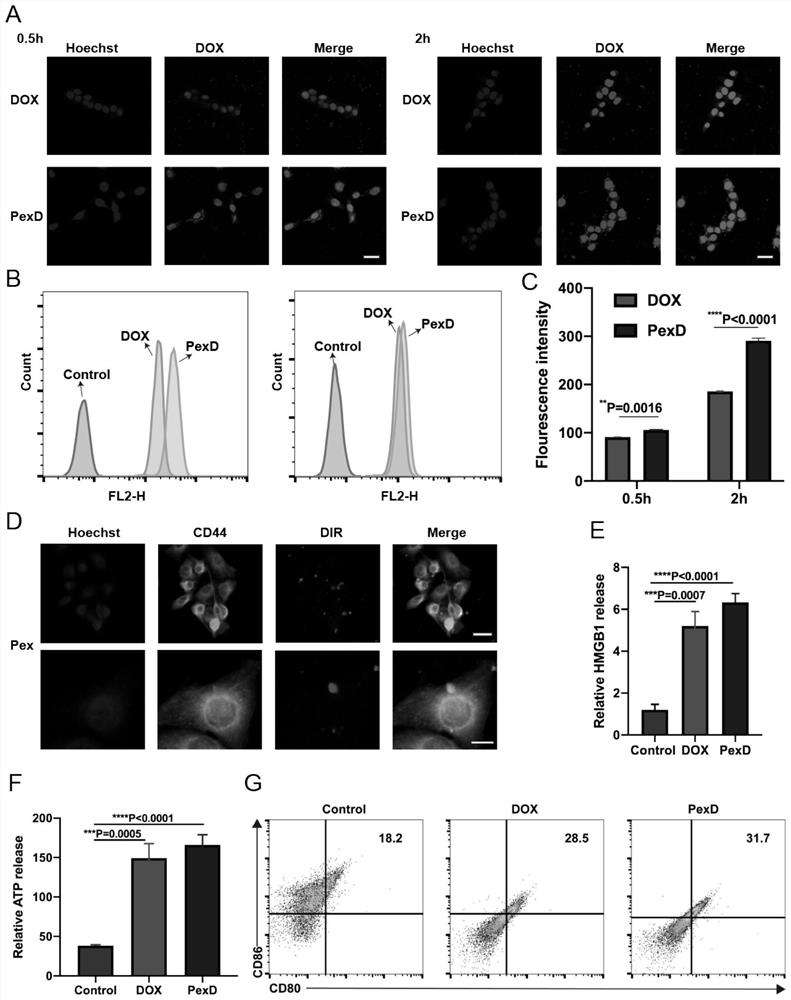
[0106] We explored the adhesion between DiR-labeled Pex and B16-F10 cells by confocal laser scanning microscopy and observed the co-localization of DiR-labeled Pex and CD44 in B16-F10 cells ( image 3 D).
Embodiment 3
[0108] The uptake and cytotoxicity of PexD by B16-F10 cells.
[0109] We observed the cellular internalization of PexD by B16-F10 by confocal ( image 3 A), At the same incubation time, the cellular uptake efficiency of PexD is higher than that of free DOX, which may be due to the binding of Pex to B16-F10 cells. We further used flow cytometry to quantify the cellular uptake of PexD and free DOX solutions by B16-F10 cells. Such as image 3 As shown in B and 3C, PexD showed enhanced DOX uptake by B16-F10 cells compared to free DOX solution. These results are consistent with those observed by fluorescence microscopy.
[0110] The in vitro cytotoxicity of PexD and free DOX solutions on B16-F10 cells was determined using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay of. Compared with free DOX solution, PexD had significantly higher cytotoxicity ( Figure 10 ). After calculation, the half maximal inhibitory concentration (IC) of PexD 50 ) values...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com