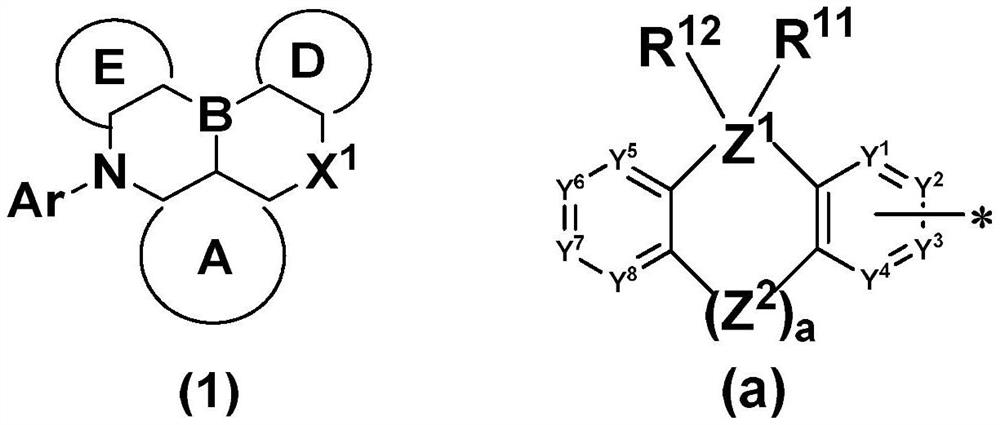
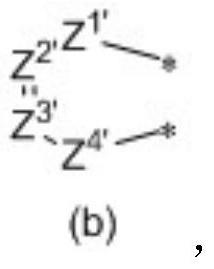
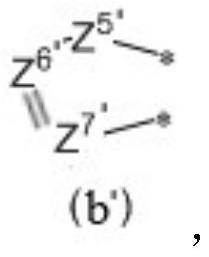Organic compound and application thereof, and organic electroluminescent device comprising organic compound
An organic compound, an unsubstituted technology, applied in the field of organic electroluminescent devices, can solve the problems of OLED product efficiency, lifespan, cost, etc., achieve low lighting voltage, improve light color purity, improve voltage and efficiency Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0095] Synthesis of V9
[0096]
[0097] Intermediate 1-1:
[0098] At room temperature, add raw material A (20g, 83.36mmol), raw material B (59.78g, 175.05mmol), Pd132 (19.48g, 25.01mmol), sodium tert-butoxide (16.02g, 166.72mmol), di Toluene (600ml) was used as a solvent, nitrogen was replaced three times, the temperature was raised to 120°C for 12 hours, then heating was stopped, filtered and evaporated to dryness, purified by column chromatography (petroleum ether:dichloromethane=10:1), and 51.0g of the product was obtained. Molecular mass determined by mass spectrometry: 804.6 (theoretical: 804.4).
[0099] Material V9:
[0100] Intermediate 1-1 (51g, 63.35mmol) was dissolved in 500ml of xylene, and n-butyllithium (76.0ml, 190.05mmol) was slowly added dropwise at -40°C. Heat and activate at ℃ for 2 hours, then cool down to -40℃, add BBr slowly 3 (45.03g, 158.4mmol), and DIEA (37.2g, 253.4mmol), heated at 110°C for 10h after returning to room temperature, then stopp...
Synthetic example 2
[0102] Synthesis of V18
[0103]
[0104] The synthesis method was the same as that of Synthesis Example 1, except that the raw material B was replaced with an equivalent amount of C to obtain 15.4 g of the product. Molecular mass determined by mass spectrometry: 862.9 (theoretical: 862.5).
Synthetic example 3
[0106] Synthesis of V19
[0107]
[0108] The synthesis method is the same as that of Synthesis Example 1, except that the raw material B is replaced with an equivalent amount of D. 11.6 g of product was obtained. Molecular mass determined by mass spectrometry: 810.8 (theoretical: 810.4).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com