Cell scaffold and construction method and application thereof
A cell scaffold and cell technology, applied in the field of biomedicine, can solve the problems of limited application, high cost and maintenance of 3D printing equipment, complicated operation, etc., and achieve the effects of simple operation, rapid preparation and short cycle.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] In this example, cavernous mesenchymal cells were isolated.
[0042] Separation of human corpus cavernosum tissue, the corpus cavernosum tissue obtained by surgery, rinsed with PBS 3 times to remove blood, and then cut into 2mm × 2mm size block tissue, using digestive enzymes (including 4mg / mL type IV collagenase, 4mg / mL collagenase Type I collagenase, 3 mg / mL hyaluronidase and 1.5 mg / mL trypsin) were digested for 30 min, and then the mass tissue was removed with a tissue strainer, and the cell components were retained by centrifugation to obtain mesenchymal cells.
Embodiment 2
[0044] In this example, cell scaffolds were constructed and identified.
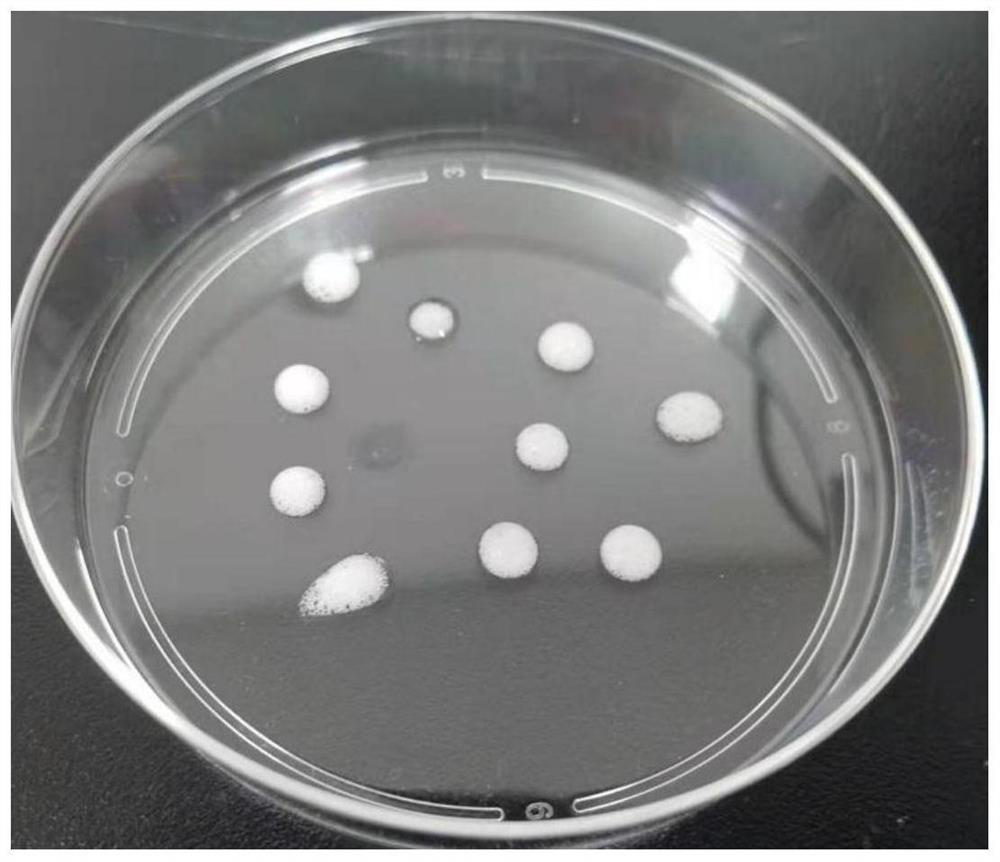
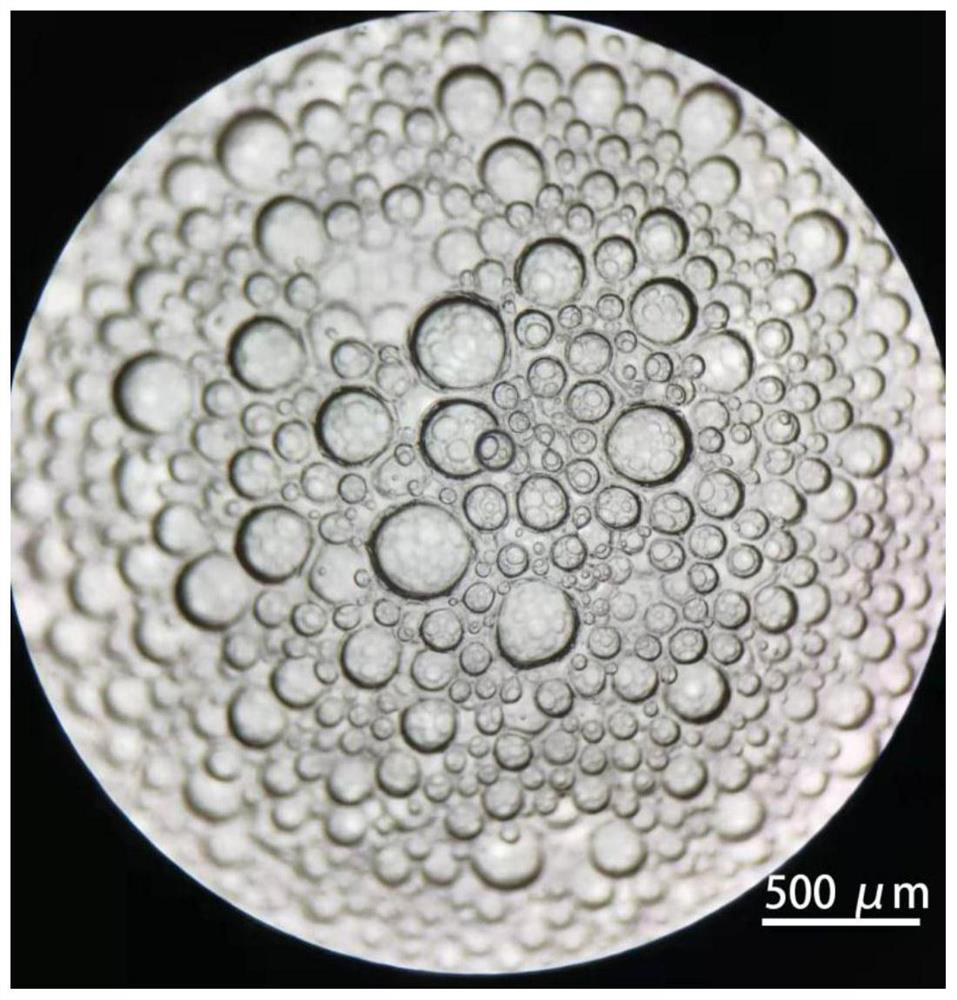
[0045] Take the mesenchymal cells isolated in Example 1, resuspend them in DMEM medium with 10% fetal bovine serum, and culture them. When the cells grow to 100% density, digest the cells to obtain a cell suspension, rinse and centrifuge with PBS, and place in Pre-cool on ice for 1 min, then use Matrigel ( 356231) resuspended at a density of 1×10 8 cells / mL, transfer 100 μL of the cell suspension to a pre-cooled 1.5mLEP tube, then immediately vortex the cell suspension, and at the same time use a 100mL pipette gun to repeatedly blow to form a large amount of foam, each time draw about 100mL of foam and place it in the on the dish (such as figure 1 Shown), under the stereoscopic microscope to observe the foam, microbubbles with a diameter of 50-500 μm can be seen ( figure 2 ), then immediately place the culture dish upside down in a 37°C incubator and wait for the matrigel to solidify. After 30 minut...
Embodiment 3
[0048] In this example, the cell scaffold prepared in Example 2 was used to culture cavernous endothelial cells.
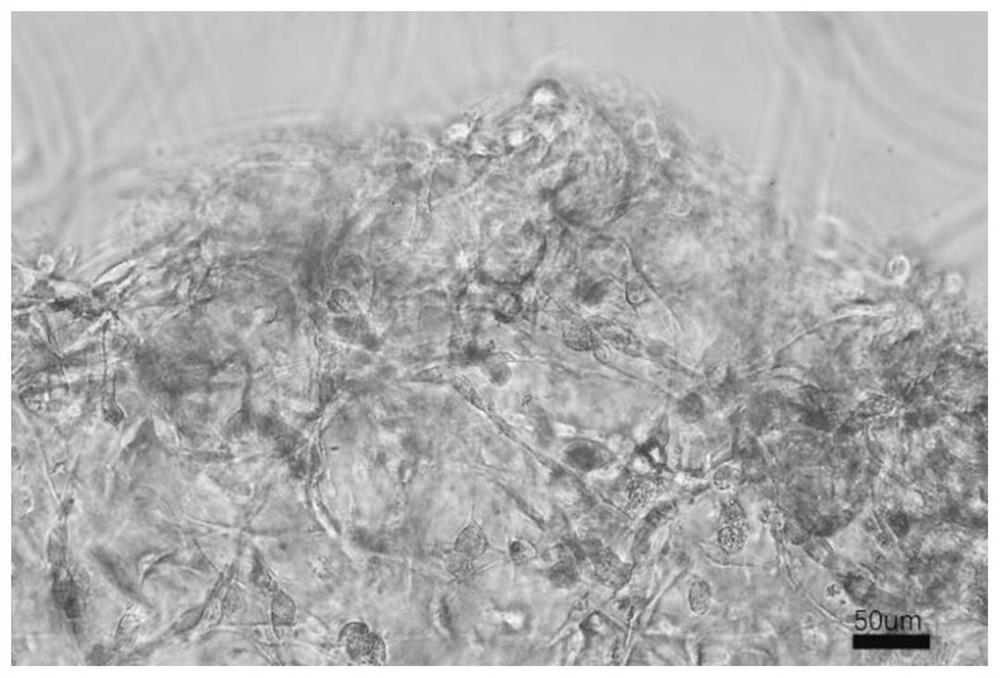
[0049] Fresh corpus cavernosum tissue was obtained surgically, digested with digestive enzymes (4mg / mL type IV collagenase, 4mg / mL type I collagenase, 3mg / mL hyaluronidase and 1.5mg / mL trypsin) for 30min, and then Use a tissue strainer to remove chunks of tissue, centrifuge to retain cell components, resuspend in endothelial cell culture medium (Lonza, EGM-2) and culture, and after 7 days, confirm the endothelial cell and smooth muscle cell colonies under the microscope according to the cell morphology, and digest them separately After forming a cell suspension, add the cell scaffold in Example 2 for co-culture, observe the colonization and growth of endothelial cells and smooth muscle in the scaffold after 2 days, mark smooth muscle cells with SAM, mark endothelial cells with CD31, and mark all nuclei with DNA staining. Separate observation and integrated observa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com