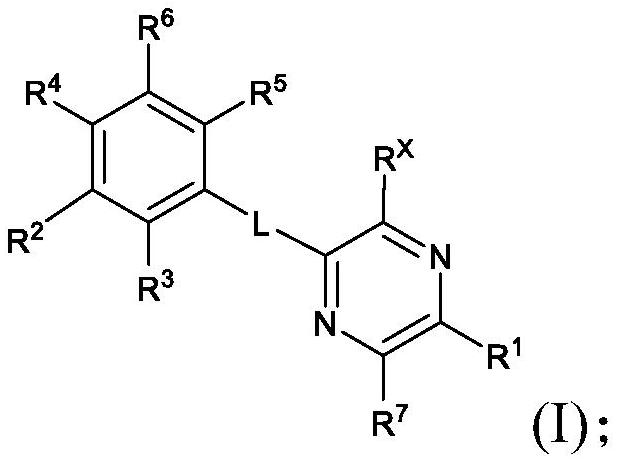
KIF18A inhibitors
A technology of -NR8-, -NR11SO2NR11, applied in the field of pharmaceutical reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0321] Ring Ar 1 Compound preparation: Ring Ar 1 An example of a compound includes Compound D-1, having the formula:
[0322]
[0323] In step D-1, compound D-1 (wherein W 4 is halogen, such as fluorine or chlorine) and R 2 The reagents react to form compound D-2. Examples of compound D-1 include, but are not limited to, 3-fluorobenzoic acid or 3-fluoro-3-methylbenzoic acid. R 2 Examples of reagents include, but are not limited to, (1) (R)-2-methylmorpholine, (2) 4,4-difluoropiperidine hydrochloride, or (3) 3,3-difluoroazetidine alkane hydrochloride. Examples of bases include, but are not limited to, diisopropylethylamine, potassium carbonate.
[0324] Step D-2: Ring Ar 2 Compound preparation:
[0325]
[0326] In step D-2, compound D-3 (wherein W 7 and W 8 Each of which is independently halo, such as fluorine, chlorine, bromine or iodine) and R X Reagents (e.g. (1) 6-azaspiro[2.5]octane hydrochloride, (2) 4,4-dimethylpiperidine hydrochloride, (3) 3,4,4-tri...
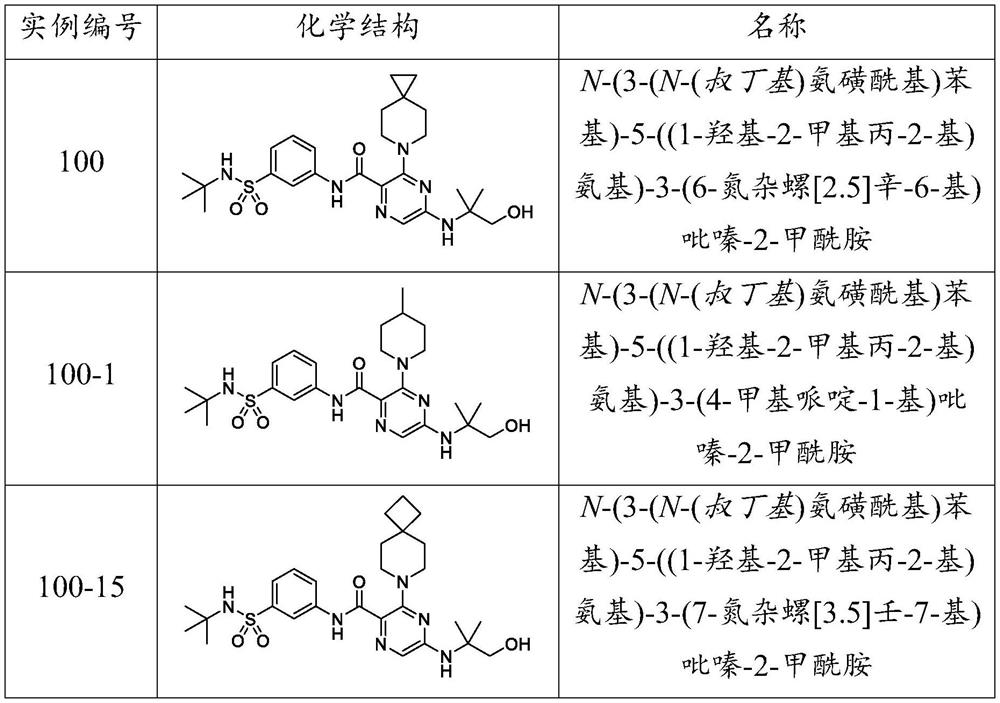
example 100
[0404] Example 100: N-(3-(N-(tert-Butyl)sulfamoyl)phenyl)-5-((1-hydroxy-2-methylpropan-2-yl)amino)-3-(6- Azaspiro[2.5]oct-6-yl)pyrazine-2-carboxamide.
[0405]
[0406] Step 1: N-(3-(N-(tert-butyl)sulfamoyl)phenyl)-3,5-dichloropyrazine-2-carboxamide (3.71g, 9.20mmol, intermediate 10), A mixture of DIPEA (2.12 mL, 11.96 mmol) and 2-amino-2-methyl-1-propanol (0.97 mL, 10.12 mmol) in ACN:DMSO (4:1, 50 mL) was stirred at RT for 16 h. Then, water was added and extracted with DCM (2x20 mL). The combined organic extracts were dried over sodium sulfate, filtered, and concentrated under vacuum. The crude product was purified by silica gel chromatography (0-30% EtOAc / EtOH (3:1) in heptane) to give N-(3-(N-(tert-butyl)sulfamoyl)phenyl) -5-Chloro-3-((1-hydroxy-2-methylpropan-2-yl)amino)pyrazine-2-carboxamide (1.50 g, 3.29 mmol, 36% yield). 1 H NMR (DMSO-d 6 )δ:10.85(s,1H),9.03(s,1H),8.44(s,1H),7.87-7.94(m,2H),7.50-7.61(m,3H),5.05(t,J=5.5Hz , 1H), 3.53 (d, J=5.5Hz, 2H), 1.39 (s, 6...
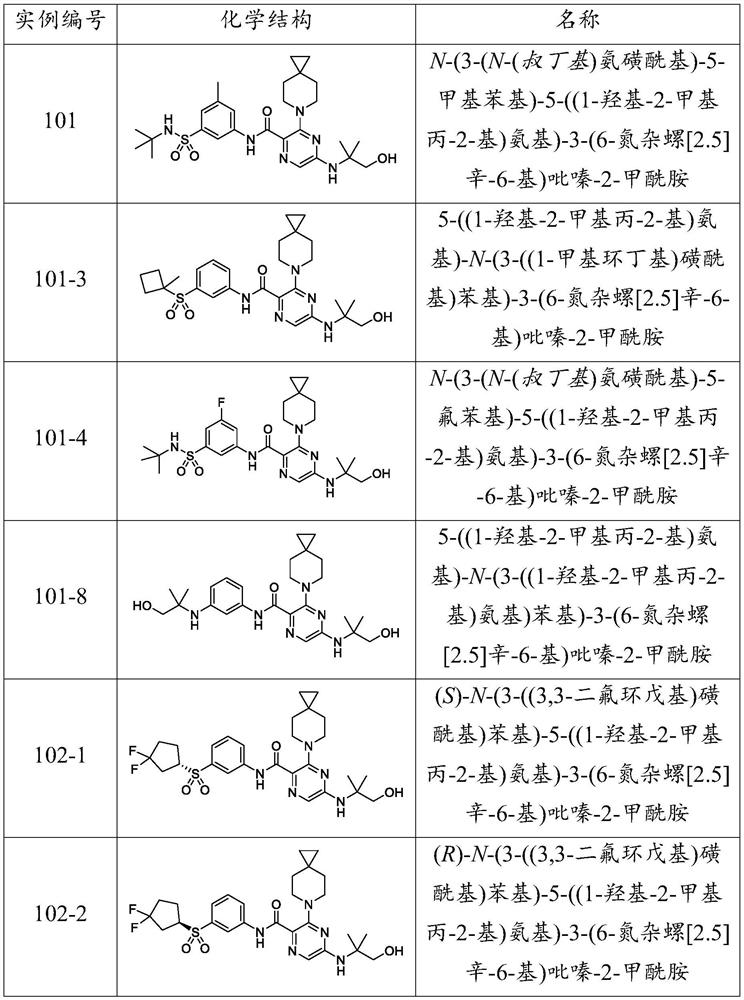
example 101
[0415] Example 101: N-(3-(N-(tert-Butyl)sulfamoyl)-5-methylphenyl)-5-((1-hydroxy-2-methylpropan-2-yl)amino)- 3-(6-azaspiro[2.5]oct-6-yl)pyrazine-2-carboxamide.
[0416]
[0417] Step 1: Add 5-(4,4-dimethyl-2-oxooxazolidin-3-yl)-3-(6-azaspiro[2.5]oct-6 to a 50-mL round bottom flask -yl)pyrazine-2-carboxylic acid (111 mg, 0.320 mmol, Intermediate 8) and DCM (4 mL). Then oxalyl chloride (0.24 mL, 0.48 mmol, 2M in DCM) was added, followed by two drops of DMF. The reaction mixture was stirred at RT for 30 min and the solvent was removed under vacuum. The residue was redissolved in DCM (4 mL) and washed with 3-amino-N-(tert-butyl)-5-methylbenzenesulfonamide (78 mg, 0.32 mmol, Intermediate 1) and DIPEA (0.17 mL, 0.96 mmol )deal with. The reaction mixture was stirred at RT for 18 h and the solvent was removed under vacuum. The crude material was absorbed onto a plug of silica gel and purified by chromatography through a silica gel column (eluting with a gradient of 0%-50% EtOA...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com