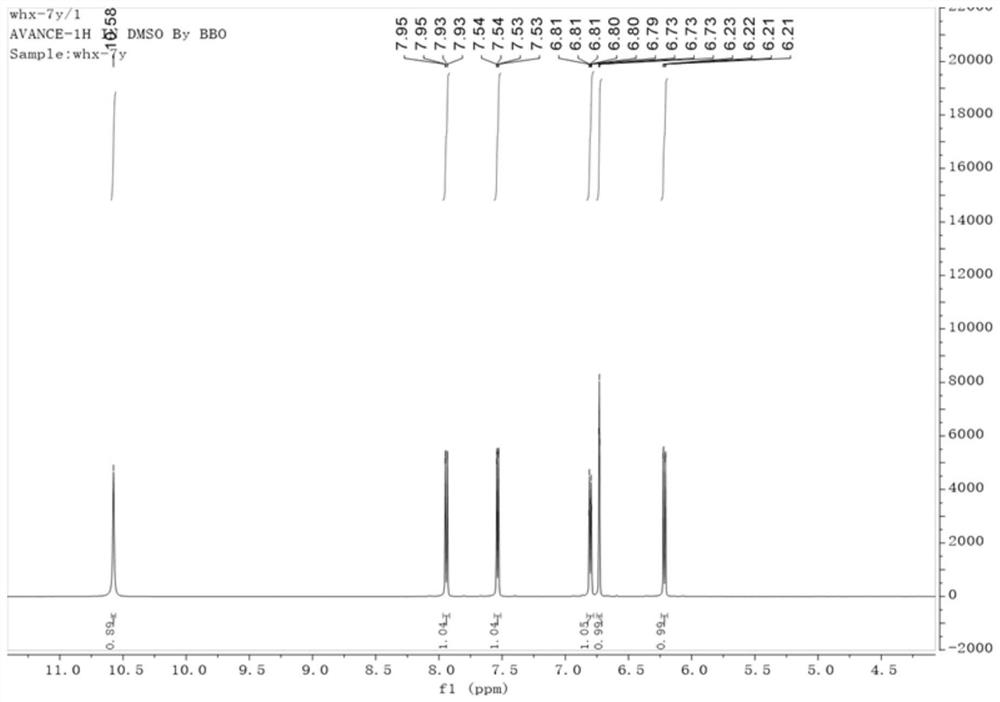
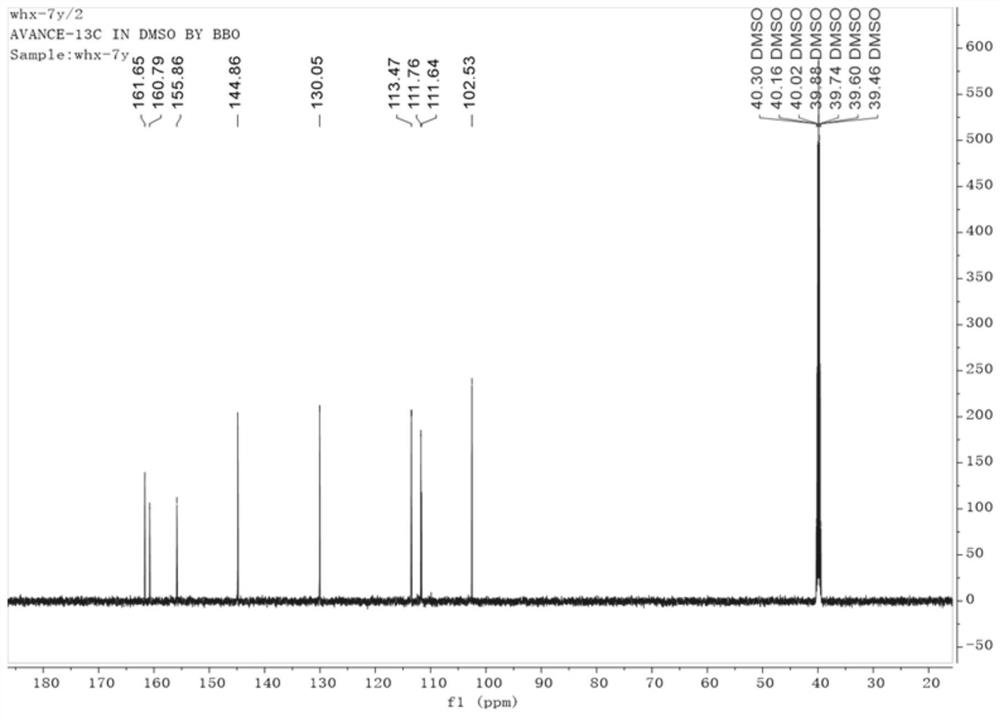
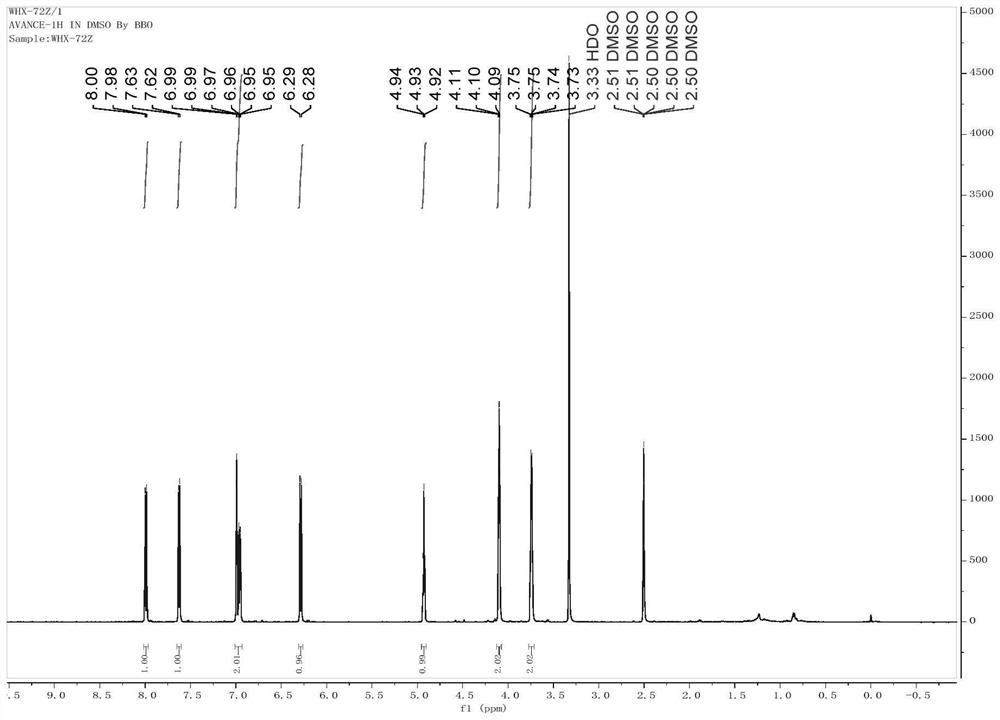
Coumarin compound and synthesis method thereof
A technology for coumarins and synthesis methods, which can be applied in the directions of organic chemistry, drug combination, antitumor drugs, etc., can solve the problems of high cost of synthesis process, harmful environment, low yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The synthetic method of the 7-hydroxycoumarin derivative of the present embodiment is as follows:
[0046] Synthesis of step 1, 7-hydroxycoumarin derivative intermediate (a1, a2, a3, a4)
[0047] Get 3.08mmol of 7-hydroxycoumarin and respectively place reaction flask with 2-bromoethanol, 3-bromo-1-n-propanol, 6-bromo-1-n-hexanol and 10-bromo-1-n-decanol, respectively Add 15.4mmol of potassium carbonate, 3.08mmol of sodium iodide, 1.54mmol of tetrabutylammonium fluoride in sequence, and then add 30mL of acetone respectively, and heat for 6 hours at a reaction temperature of 56°C respectively. TLC monitors reaction respectively, and the developing agent used in monitoring is V (petroleum ether): V (ethyl acetate)=10:5, (by R fThe value is judged between 1.2-1.5, and the follow-up operation is carried out) After the reaction is completed, filter respectively, and distill under reduced pressure to obtain yellow oil (38-45° C.). Separation and purification through silica g...
Embodiment 2
[0096] The synthetic method of the 4-hydroxycoumarin derivative of the present embodiment is:
[0097] Synthesis of step 1, 4-hydroxycoumarin derivative intermediate (b1, b2, b3, b4)
[0098] Get 4-hydroxycoumarin 3.08mmol and place reaction flask respectively with 2-bromoethanol, 3-bromo-1-n-propanol, 6-bromo-1-n-hexanol and 10-bromo-1-n-decanol, respectively Add 15.4mmol of potassium carbonate, 3.08mmol of sodium iodide, 1.54mmol of tetrabutylammonium fluoride in sequence, and then add 30mL of acetone respectively, and heat for 6 hours at a reaction temperature of 56°C respectively. TLC follow-up monitoring respectively, developer is V (petroleum ether): V (ethyl acetate)=10:5 (by R f The value is between 1.2-1.5 to determine the follow-up operation), after the reaction is completed, filter respectively, and distill under reduced pressure to obtain viscous yellow oil (38-45°C). Separation and purification through silica gel column by dry method respectively, eluent V (petr...
Embodiment 3
[0149] The synthetic method of the 4-methyl-7-hydroxycoumarin derivative of the present embodiment is:
[0150] Step 1, the synthesis of 4-methyl-7-hydroxycoumarin derivative intermediate (c1, c2, c3, c4)
[0151] Get 2.84mmol of 4-methyl-7-hydroxycoumarin with 2-bromoethanol, 3-bromo-1-n-propanol, 6-bromo-1-n-hexanol, 10-bromo-1-n-decanol In the reaction bottle, 14.20 mmol potassium carbonate, 2.84 mmol sodium iodide, 1.42 mmol tetrabutylammonium fluoride were added in turn, and then 30 ml acetone were added respectively, and the reaction temperature was 56° C. respectively, and heated for 6 hours. TLC monitors reaction respectively, developing agent is V (petroleum ether): V (ethyl acetate)=10:5 (by R f The value is between 1.2-1.5 to determine the follow-up operation), after the reaction is completed, filter respectively, and distill under reduced pressure to obtain viscous yellow oil (38-45°C). Separation and purification through silica gel column by dry method, eluent V...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com