Protein probe and application thereof in detecting BACE1 activity
A protein and probe technology, applied in the field of biomedicine, can solve the problems of unable to restore the physiological environment, complex probe synthesis, cumbersome data processing, etc., to avoid the interference of non-specific signals, simple and direct operation, and low detection equipment requirements. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
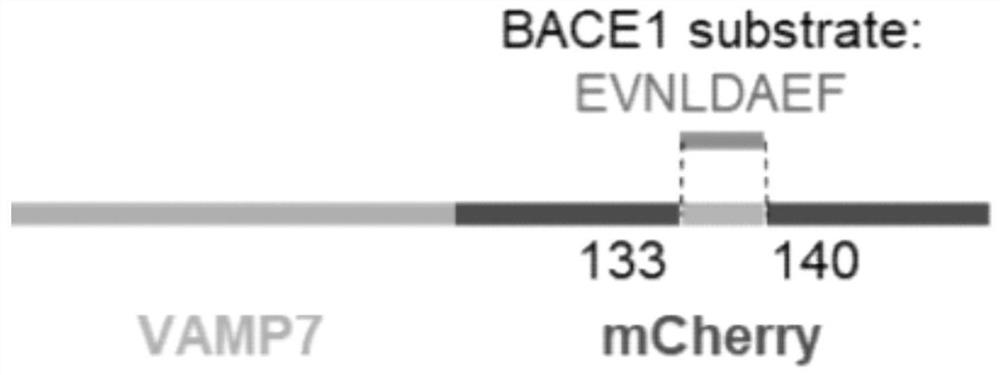
[0094] Embodiment 1 Fluorescent protein probe is constructed to pcDNA3.1
[0095] In this embodiment, fluorescent protein probes were constructed into pcDNA3.1, and the constructed probes were expressed in HEK293T cells.
[0096] Such as Figure 4 The structure diagram of the constructed plasmid is shown below, and the operation steps are described in detail below:
[0097] Use the PCR method to amplify mCherry-N and mCherry-C from the plasmid of mCherry-C1, using primers:
[0098] mCherry-N-for:5'-CTTGGTACCGAGCTCGGATCCATGGTGAGCAAGGGCGAGGAGGAT-3'(BamHI) (as shown in SEQ ID NO:1);
[0099] mCherry-N-rev: 5'-GGTGCCGCGCAGCTT-3' (as shown in SEQ ID NO: 2);
[0100] mCherry-C-for:5'-AAGCTGCGCGGCACCGAAGTGAATCTGGATGCAGAATTCCGACAGAAGAAGACCATGGGCTGGGAG-3' (as shown in SEQ ID NO:3);
[0101] mCherry-N-rev: 5'-CCACACTGGACTAGTGGATCCCTACTTGTACAGCTCGTCCATGCCGCC-3' (BamHI) (shown in SEQ ID NO: 4).
[0102] Wherein mCherry-C-for contains the base sequence (5'-GAAGTGAATCTGGATGCAGAATTCCGA-3'...
Embodiment 2
[0110] Example 2 Optimized screening of polypeptide sequences recognized by BACE1
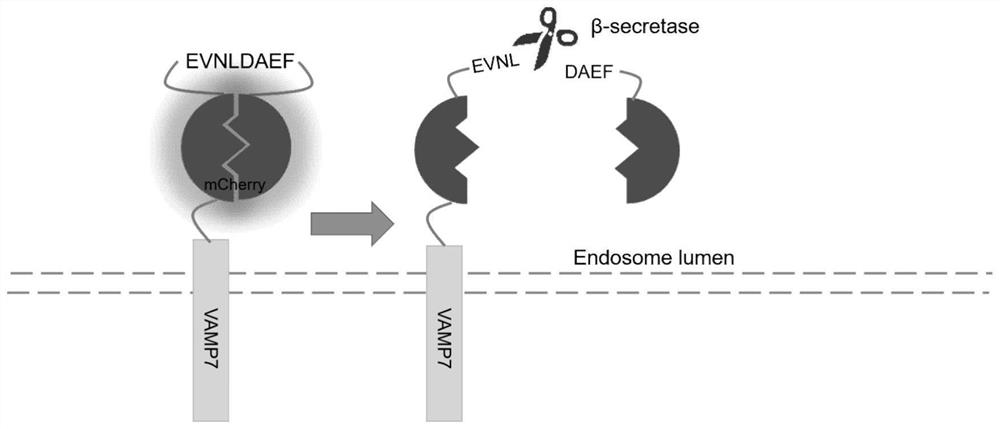
[0111] During the construction of the probe, optimized screening was carried out for the peptide sequence recognized by BACE1:
[0112] The original 133-141: EVNLDAEFR was shortened to construct 133-140: EVNLDAEF, 134-139: VNLDAE, 134-140: VNLDAEF, 134-141: VNLDAEFR, 135-140: NLDAEF. Subsequently, a total of 6 probes were tested for fluorescence intensity and sensitivity:
[0113] The result is as Figure 9 and as shown in Table 1, Figure 9 The middle left image is the confocal image of the fluorescence intensity, Figure 9 The upper right figure shows the basic fluorescence intensities of the six probes, Figure 9 The bottom right panel shows the sensitivity of the six probes. On the whole, the sensitivity of 133-140 and 134-140 has been improved, and the fluorescence intensity has also been improved, so three kinds of peptide sequences were screened out.
[0114] Table 1
[0115]
...
Embodiment 3
[0123] Example 3 Study on the change of BACE1 activity in cell senescence
[0124] Alzheimer's disease (AD) is a neurodegenerative disease, and the occurrence and development of AD are closely related to the growth of age. BACE1 is directly related to the accumulation of Aβ, and plays an important role in the occurrence and development of AD. The purpose of this example is to use the probe of the present invention to study the change of BACE1 activity in cell senescence.
[0125] For this purpose, HEK293T cells at passage 19 and passage 69 were transfected into mCherry-BACE1-sub, and confocal imaging was performed after culture. The result is as Figure 7 As shown in Table 3, with the increase of the number of HEK293T generations, the fluorescence intensity of the probe weakened, and the activity of BACE1 continued to increase. Therefore, we can reasonably speculate that the enhanced activity of BACE1 leads to more accumulation of Aβ in brain tissue, leading to the death of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com