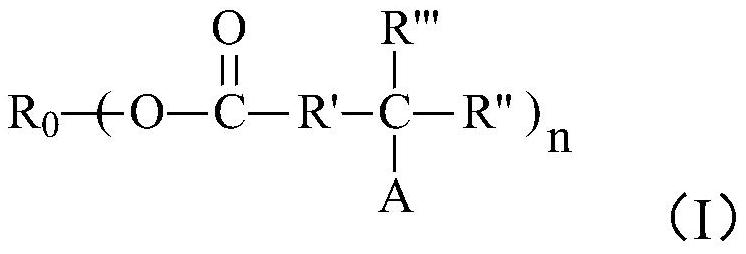Ester compound, preparation method and application thereof, and antioxidant composition
A technology of ester compounds and compounds, applied in the preparation of organic compounds, cyanide reaction preparation, lubricating compositions, etc., can solve the problems of low product yield, easy precipitation, poor oil solubility, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
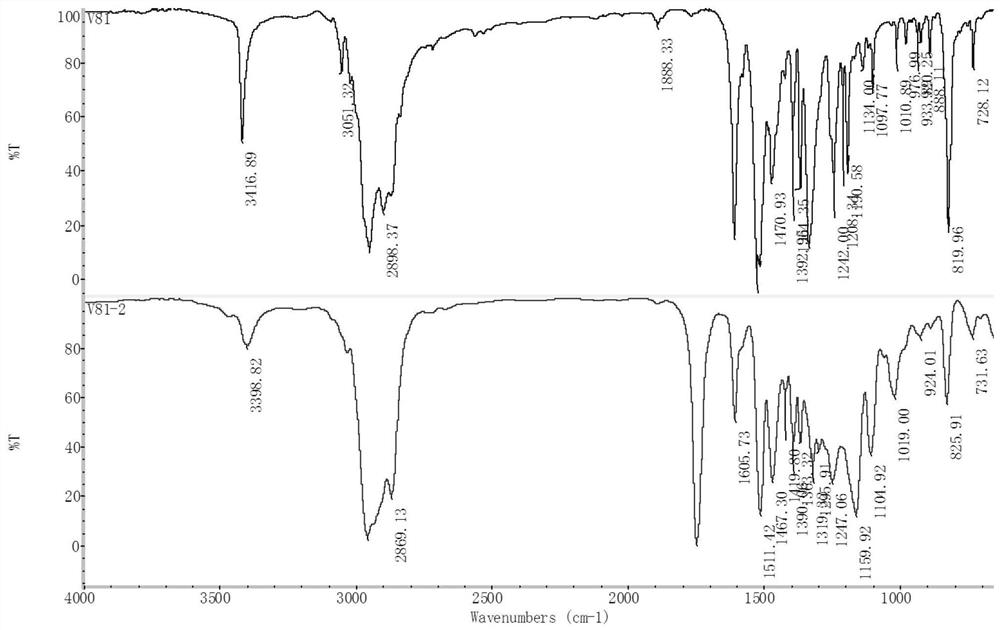
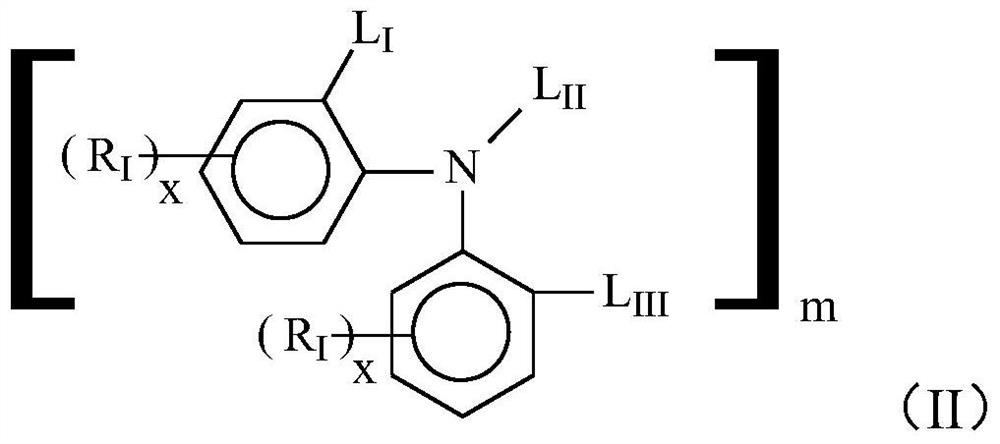
[0109] Add 117g raw material 4,4'-diisooctyl-diphenylamine (VANLUBEV81) to 117g 100℃ kinematic viscosity=3.8mm 2 / s Trimethylolpropane saturated fatty acid ester, heat, stir and dissolve the mixed system in the presence of nitrogen, maintain the mixed system at 145°C, add 84g of di-tert-butyl peroxide to the reaction system, and react at a constant temperature of 145°C 4h, then distill under reduced pressure at 145°C and ≤1000Pa for 30min, then increase the vacuum degree to ≤500Pa, and gradually raise the temperature to and maintain at 175°C for more than 40min under reduced pressure distillation. After the vacuum distillation is completed, the product is cooled under a nitrogen environment , to finally obtain 225g of reaction product A1, the reaction product A1 mainly contains compounds of structural formula P-1, structural formula P-2, structural formula P-3 and a small amount of structural formula P-4, structural formula P-5, structural formula P-6, The compound of structur...
Embodiment 2
[0122] Add 120g raw material 4,4'-diisooctyl-diphenylamine to 120g 100℃ kinematic viscosity=5.02mm 2 / s Heat, stir and dissolve the mixed system in the pentaerythritol ester in the presence of nitrogen, maintain the mixed system at 140°C, add 88g of di-tert-butyl peroxide to the reaction system, and carry out the reaction at 140°C for 3h, then at 140°C, Distill under reduced pressure at ≤1000Pa for 30min, then raise the vacuum degree to ≤500Pa, and at the same time gradually raise the temperature to and maintain at 175°C for more than 40min under reduced pressure distillation. Product A2, the reaction product A2 mainly comprises ester compounds of structures similar to structural formula P-1, structural formula P-2, and structural formula P-3 (the difference is that the ester group in the ester compound in this embodiment is pentaerythritol ester Ester group), while containing a small amount of compounds of structural formula P-4, structural formula P-5, structural formula P-6,...
Embodiment 3
[0124] Add 120g raw material 4,4'-diisooctyl-diphenylamine to 120g kinematic viscosity=5mm 2 / s mixed polyol saturated ester (wherein the mass ratio of trimethylolpropane saturated fatty acid ester and dipentaerythritol ester is 3:1), in the presence of nitrogen, the mixed system is heated and stirred, dissolved, and the mixed system is maintained at 150°C, add 72g of di-tert-butyl peroxide to the reaction system, react at 150°C for 4h, then distill under reduced pressure at 150°C and ≤1000Pa for more than 40min, after the vacuum distillation is completed, the product is cooled under nitrogen atmosphere , finally obtain 235g reaction product A3; Mainly comprise the compound of structural formula P-1, structural formula P-2, structural formula P-3 similar structure in the reaction product A3 (the difference is that the ester group in the ester compound in the present embodiment is The ester group of mixed polyol saturated ester) contains less compound of structural formula P-4,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Kinematic viscosity | aaaaa | aaaaa |
| Kinematic viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com