Pharmaceutical composition for treating and repairing spinal cord injury and application thereof
A technique for spinal cord injury and composition, applied in the field of pharmaceutical composition for treating and repairing spinal cord injury
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Example 1 Preparation of plasmids and constructs
[0071] (1) Rab3A and spastin sequences were obtained for preparation of cDNA, which were then cloned into pEGFP-C1 (Clontech, CA, USA), pGEX-5x-3 (Amer-sham Pharmacia Biotech, NJ, USA) and pCMV-Tag2 (Stratagene, CA, USA) vector, and the constructed construct was confirmed by DNA sequencing method;
[0072] (2) Pull-down analysis using glutathione S-transferase (GST): Spinal cord tissue sections were ground and lysed, then GST-agarose beads (Invitrogen, CA, USA) were rinsed and mixed with lysis buffer at 4 Incubate for 1 h at °C, then centrifuge at 1000 g for 10 min. 4°C. The supernatant was then collected and these steps were repeated one more time. Appropriate bead fusion proteins were then added to the spinal cord tissue, and the samples were incubated overnight at 4°C. After spinning at 1000 g for 5 min at 4°C, unbound protein was washed with 1 mL of wash buffer, and then the samples were spun at 1000 g for 1 min...
Embodiment 2
[0076] Example 2 Construction of rat SCI model
[0077] The Sprague-Dawley (SD) rats used in the experiment were purchased from the Laboratory Animal Center of Sun Yat-Sen University. The rats were housed individually in a facility at 25±3°C and provided with regular food and water. Specifically, the following steps were included:
[0078] (1) SD rats were anesthetized by intraperitoneal injection of 10% chloral hydrate (0.35 mL / 100 g body weight), after which a 2.5 cm longitudinal dorsal incision was made to expose the T9-11 spinous process and lamina;
[0079] (2) The entire T10 lamina is removed, and the exposed area of the spine is about 2.5mm × 3mm;
[0080] (3) Use a stabilizer to fix the T10 section bilaterally, and set the nitrogen tank to control the impact head to 18psi or 124kPa. Load the U-shaped stabilizer with the rat onto the platform of the Louisville Injury System Equipment (LISA) and adjust the dura / spinal height directly below the impactor while monitorin...
Embodiment 3
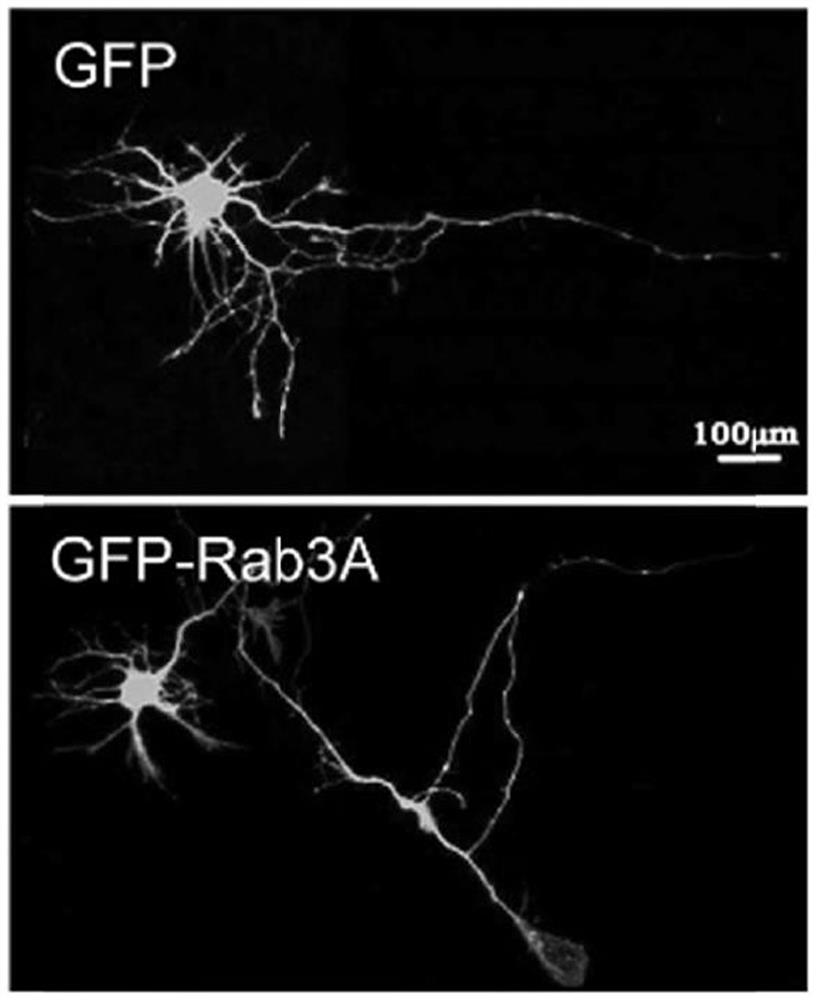
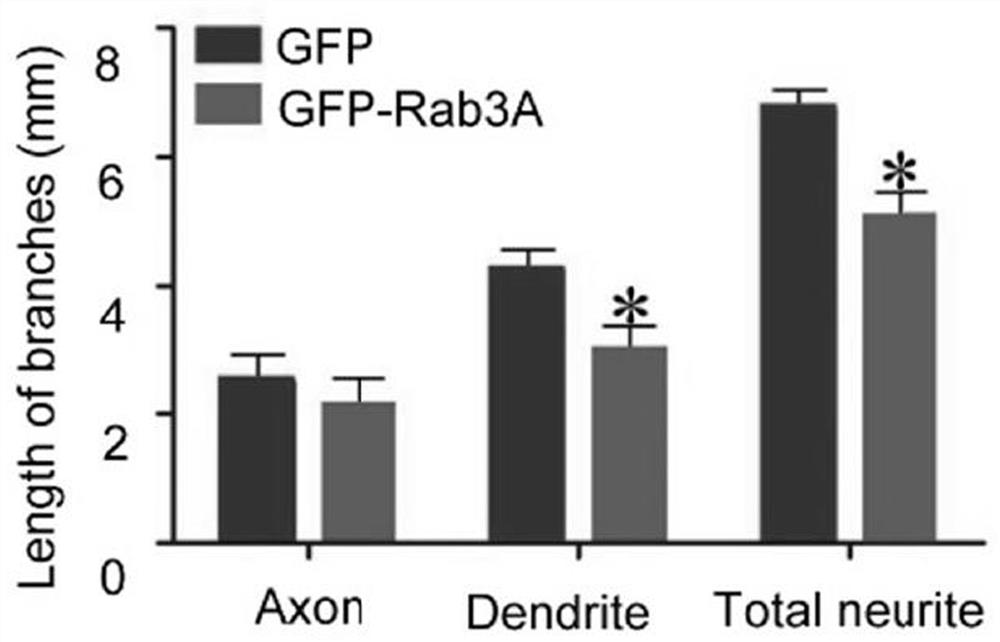
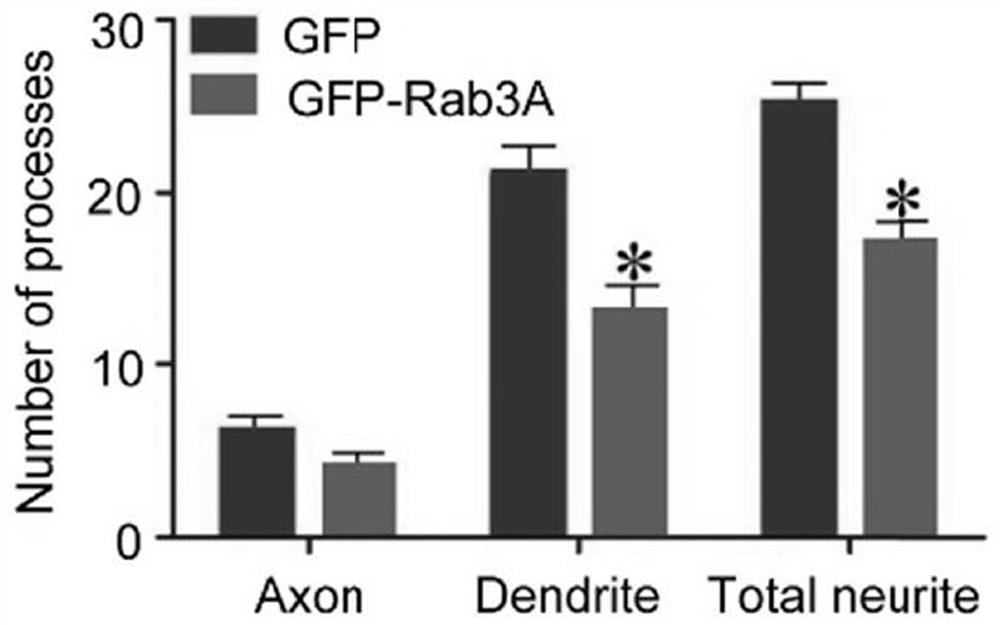
[0085] Example 3 Rab3A regulates neuron growth and development
[0086] Firstly, the research on the growth and development of neurons by Rab3A overexpression includes the following steps:
[0087] (1) GFP-Rab3A and GFP (blank control group) were transfected into primary cultured hippocampal neurons for 72 hours, respectively;
[0088] (2) After 48 hours, the hippocampal neurons were fixed and photographed, and the scale was 100 μm.
[0089] The result is as Figure 1-3 shown, where figure 1 Schematic diagram of the immunofluorescence staining results of hippocampal neurons, figure 2 Schematic diagram of the quantitative statistics of the length of axons and neurites in hippocampal neurons, image 3 Schematic diagram of the quantitative statistical results of the number of axons and processes in hippocampal neurons, n=20 / group, the results are represented by Mean±SD, and * represents p <0.05. The scale bar is 100 μm. According to the results, overexpression of Rab3A in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com