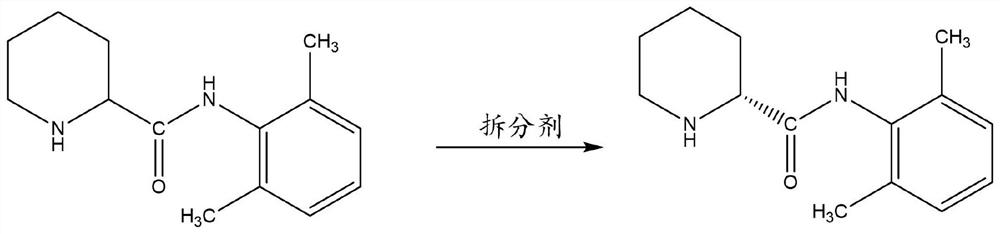
Method for preparing ropivacaine hydrochloride impurity (R)-N-(2, 6-dimethylphenyl) piperidine-2-formamide
A technology of ropivacaine hydrochloride and xylyl, which is applied in the field of preparing ropivacaine hydrochloride impurity-N-piperidine-2-carboxamide, which can solve the problems of less synthesis methods and no sales, and achieve chiral purity Good, solve the problem of quality control, the effect of controllable process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
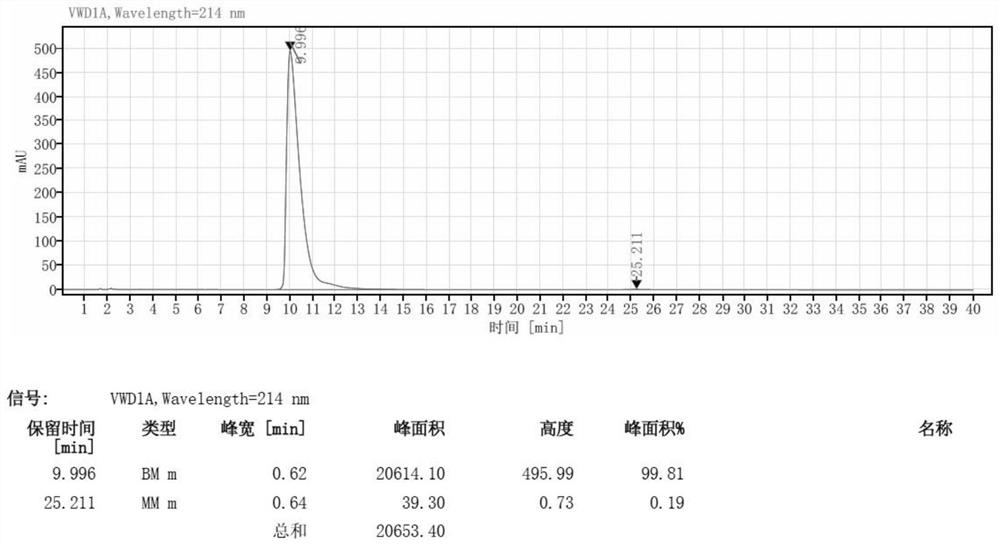
[0035] The D-type dibenzoyl tartaric acid 10g was stirred with acetone 35g to heat up to dissolve; the 20gN(2,6-xylphenyl)piperidine-2-formamide was added to the reaction bottle added to acetone 35g purified water 10g stirred to heat up to 50 °C dissolved, dropwise added benzoyl tartaric acid solution to maintain 50 °C reaction for 1h, cooled to -5 °C to analyze the sperm for 1h, filtered filtrate. The filtrate was concentrated at 50 °C under reduced pressure to no condensate, the pH was adjusted to 14 with 10% potassium hydroxide, 30g of toluene was added to the system, the organic phase was separated, and the white solid was concentrated at 50 °C to give a yield of 45% of the white solid 9g and an optical purity of 99.8%.
Embodiment 2
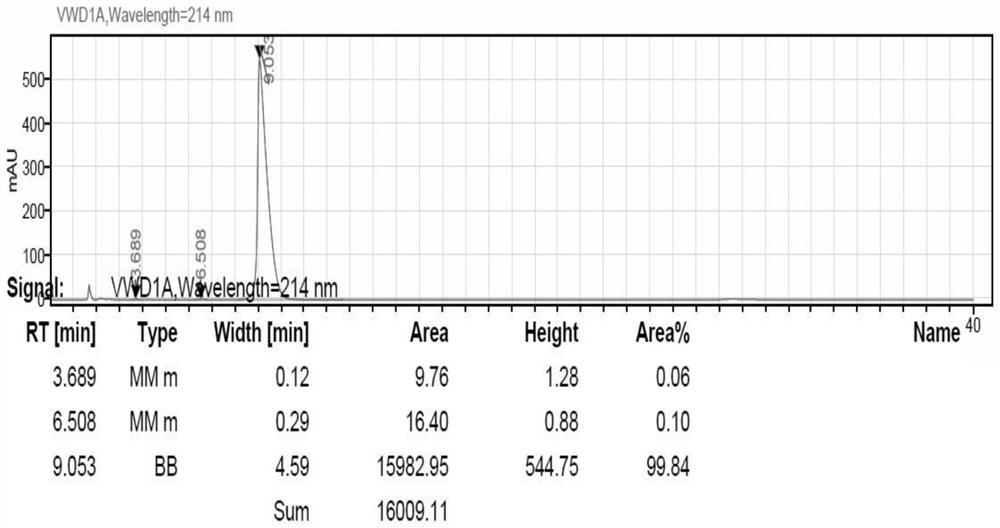
[0037] The L-type di-p-p-methyldibenzoyl tartaric acid 100g with acetone 350g stirred to heat up to dissolve; 200gN- (2,6-xylphenyl) piperidine-2-formamide was added to the reaction bottle added to acetone 350g purified water 100g stirred to heat up to 55 °C dissolved, dropwise added benzoyl tartaric acid solution to maintain 55 °C reaction for 1h, cooled to -5 °C precipitation 2h, filtered filtrate. The filtrate was concentrated at 50 °C under reduced pressure to no condensate, the pH was adjusted to 14 with 10% sodium hydroxide, 300g of toluene was added to the system, the organic phase was separated, and the white solid was concentrated at 50 °C to give a yield of 47% of the white solid 94g and an optical purity of 99.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com