Side chain piperidine cation grafting type polybiphenyl alkaline membrane and preparation method thereof
A polybiphenyl alkaline and cationic technology, used in electrochemical generators, fuel cells, electrical components, etc., to achieve the effects of excellent thermal stability and high ionic conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
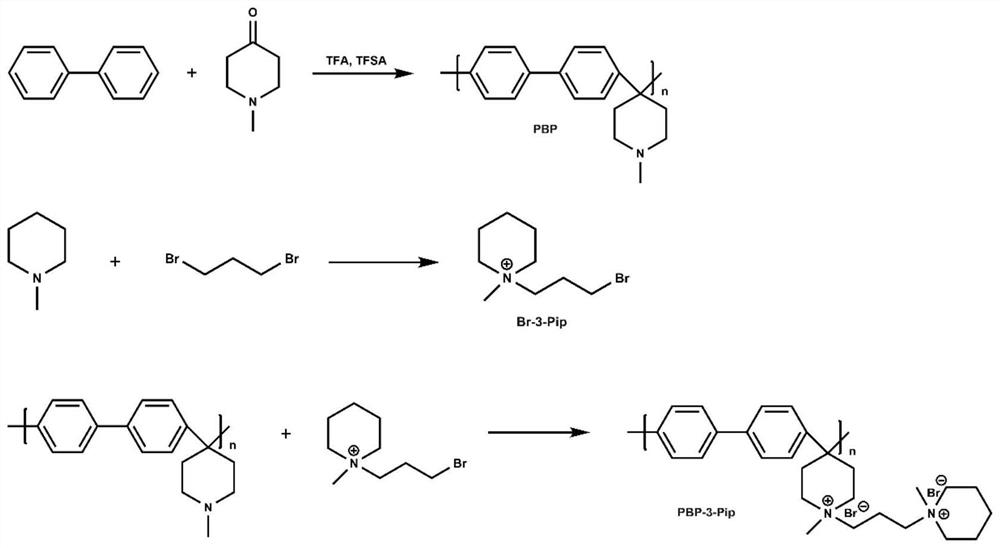
[0056] The preparation of 1-(3-bromopropyl)-1-methylpiperidine cationic grafted polybiphenyl (PBP-3-Pip) alkaline membrane, such as figure 1 As shown, it is the preparation process of 1-(3-bromopropyl)-1-methylpiperidine cationic grafted polybiphenyl (PBP-3-Pip) alkaline membrane in Example 1 of the present invention, and the specific steps are as follows :
[0057] (1) Preparation of polybiphenyl (PBP)
[0058] Biphenyl (1 g) and N-methylpiperidone (0.83 mL) were dissolved in dichloromethane (5 mL); then, a mixed liquid of trifluoromethanesulfonic acid (5 mL) and trifluoroacetic acid (0.5 mL) was added gradually. dropwise, and reacted at 0 °C for 6 h to obtain a wine-red viscous liquid. 2 CO 3 Precipitate in aqueous solution, soak at room temperature for 24 hours, and filter to obtain a white solid sample, which is fully washed with deionized water and dried to obtain polybiphenylpiperidine (PBP) polymer;
[0059] (2) Preparation of 1-(3-bromopropyl)-1-methylpiperidine (B...
Embodiment 2
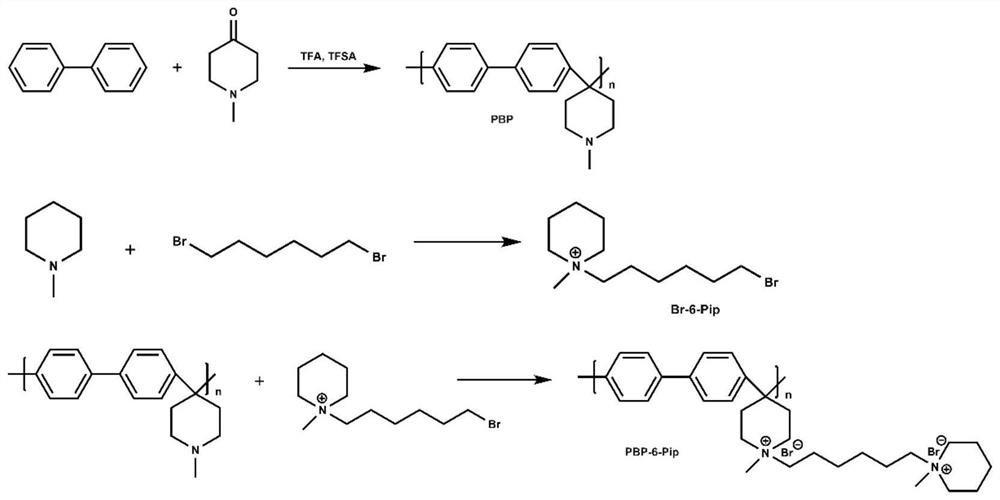
[0066] The preparation of 1-(6-bromohexyl)-1-methylpiperidine cationic grafted polybiphenyl (PBP-6-Pip) alkaline membrane, such as Figure 4-2 As shown, it is the preparation flow chart of 1-(6-bromohexyl)-1-methylpiperidine cation-grafted polybiphenyl (PBP-6-Pip) alkaline membrane in Example 2 of the present invention; the specific preparation process as follows:
[0067] (1) Preparation of polybiphenyl (PBP)
[0068] With embodiment 1;
[0069] (2) Preparation of 1-(6-bromohexyl)-1-methylpiperidine (Br-6-Pip)
[0070] 1,6-Dibromohexane (1.5 mL), N-methylpiperidine (1 mL), and ethyl acetate (20 mL) were added to a round-bottomed flask, and nitrogen was passed through to react fully to obtain a white solid precipitate, which was evacuated. Filtration, purification with ethyl acetate, removal of excess reactant, and drying to give side chain piperidine cation 1-(6-bromohexyl)-1-methylpiperidine (Br-6-Pip);
[0071] (3) Preparation of 1-(6-bromohexyl)-1-methylpiperidine cati...
Embodiment 3
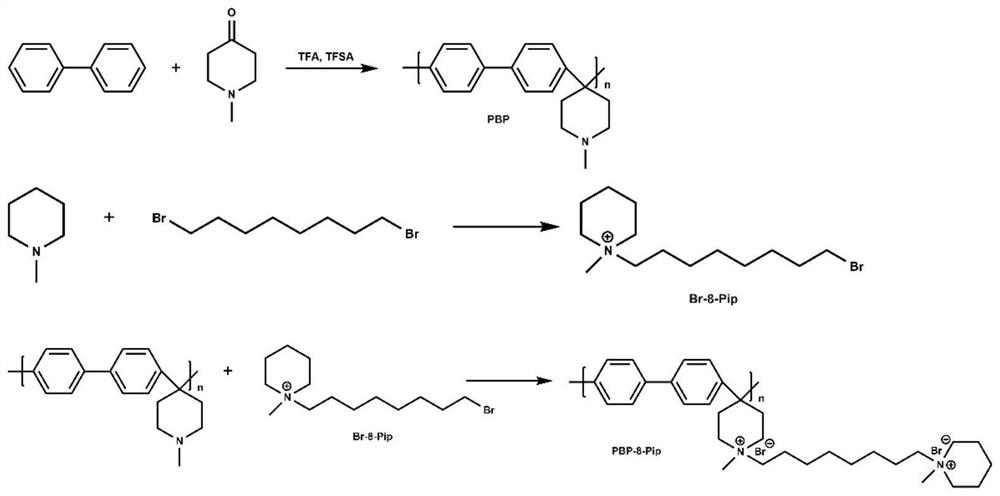
[0076] The preparation of 1-(8-bromooctyl)-1-methylpiperidine cationic grafted polybiphenyl (PBP-8-Pip) alkaline membrane, such as Figure 4-3 shown, is the preparation flow chart of 1-(8-bromooctyl)-1-methylpiperidine cation-grafted polybiphenyl (PBP-8-Pip) alkaline membrane in Example 3 of the present invention; the specific preparation The process is as follows:
[0077] (1) Preparation of polybiphenyl (PBP)
[0078] With embodiment 1;
[0079] (2) Preparation of 1-(8-bromooctyl)-1-methylpiperidine (Br-8-Pip)
[0080] 1,8-Dibromooctane (1.75 mL), N-methylpiperidine (1 mL), and ethyl acetate (20 mL) were added to a round-bottomed flask, and nitrogen was passed through to fully react to obtain a white solid precipitate. Filtration, purification with ethyl acetate, removal of excess reactant, and drying yielded the side chain piperidine cation 1-(6-bromohexyl)-1-methylpiperidine (Br-8-Pip);
[0081] (3) Preparation of 1-(8-bromooctyl)-1-methylpiperidine cationic grafted po...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Resonance frequency | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com