Solid dispersion, preparation, preparation method and application thereof
A technology of solid dispersion and carrier, which is applied in the direction of non-active ingredient medical preparations, medical preparations containing active ingredients, pill delivery, etc. It can solve the problems of low solubility and difficult preparations, and achieve low impurity content and dissolution rate Good, the effect of improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0122] Example 1: Preparation of compounds of formula (I)
[0123] The compound of formula (I) was prepared according to WO2018 / 027097A1, then the compound of formula (I) was placed in a bottle, an appropriate amount of 1,4-dioxane was added, and it was allowed to stand at room temperature for 2 days. The obtained crystal form was centrifuged and dried at room temperature. , to obtain the crystalline form of compound (I).
Embodiment 2
[0124] Example 2: Preparation of compounds of formula (I)
[0125] The compound of formula (I) was prepared according to WO2018 / 027097A1, and then the compound of formula (I) was placed in a bottle, an appropriate amount of ethanol was added, stirred at room temperature for 5 days, and centrifuged. , and dried at room temperature to obtain acetone solvate crystalline form.
Embodiment 3
[0126] Example 3: Preparation of compound of formula (I)
[0127] The compound of formula (I) was prepared according to WO2018 / 027097A1, then the compound of formula (I) was placed in a bottle, an appropriate amount of acetonitrile solvent was added, and stirred at room temperature for 5 days. The obtained crystal form was centrifuged and dried at room temperature to obtain acetonitrile solvate crystals form.
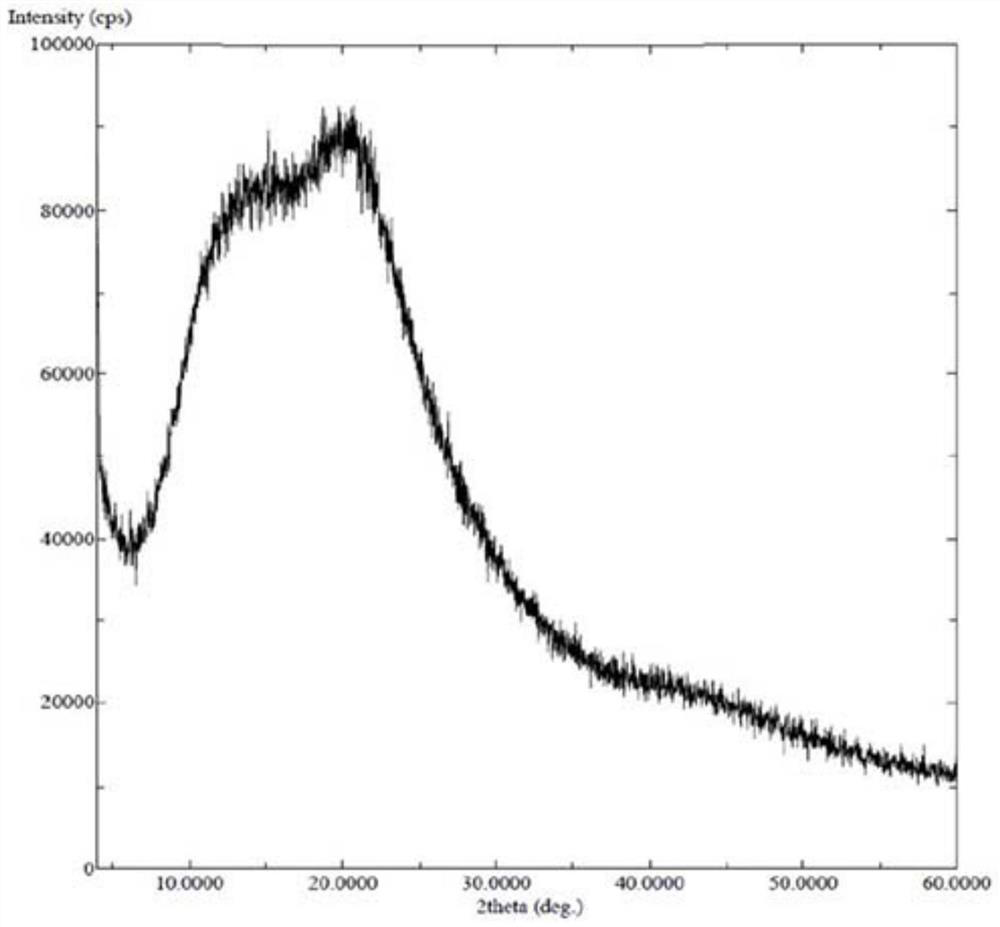
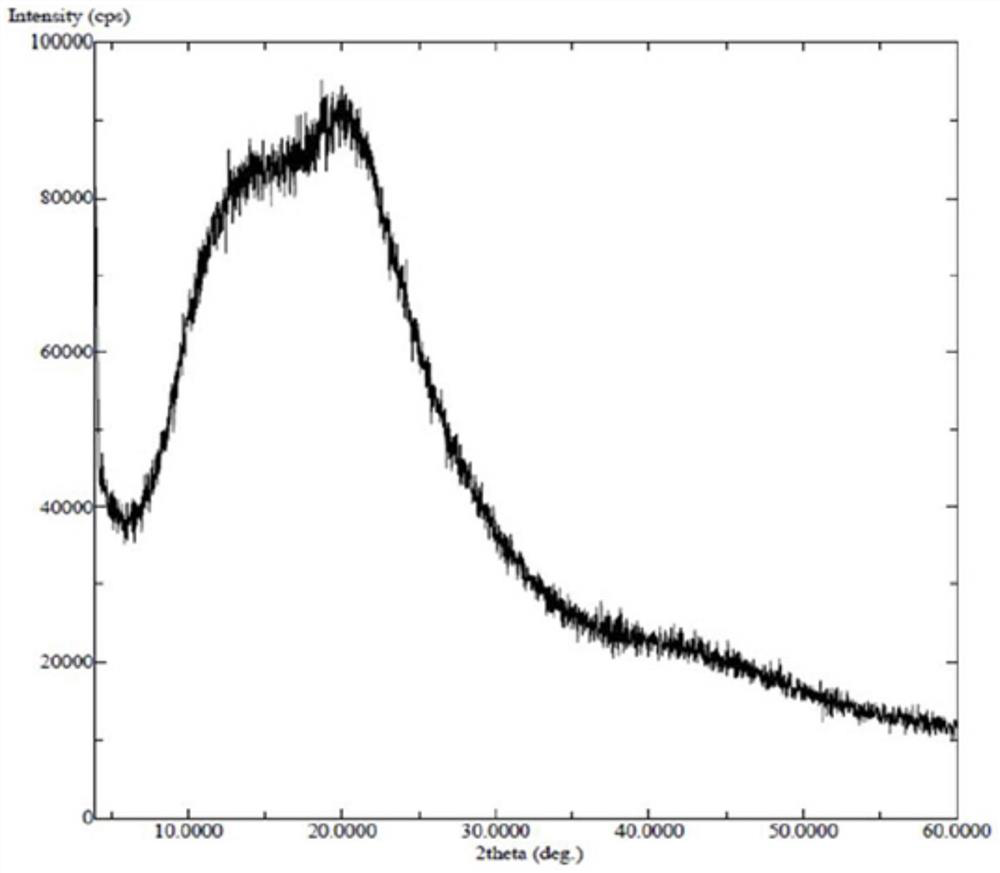
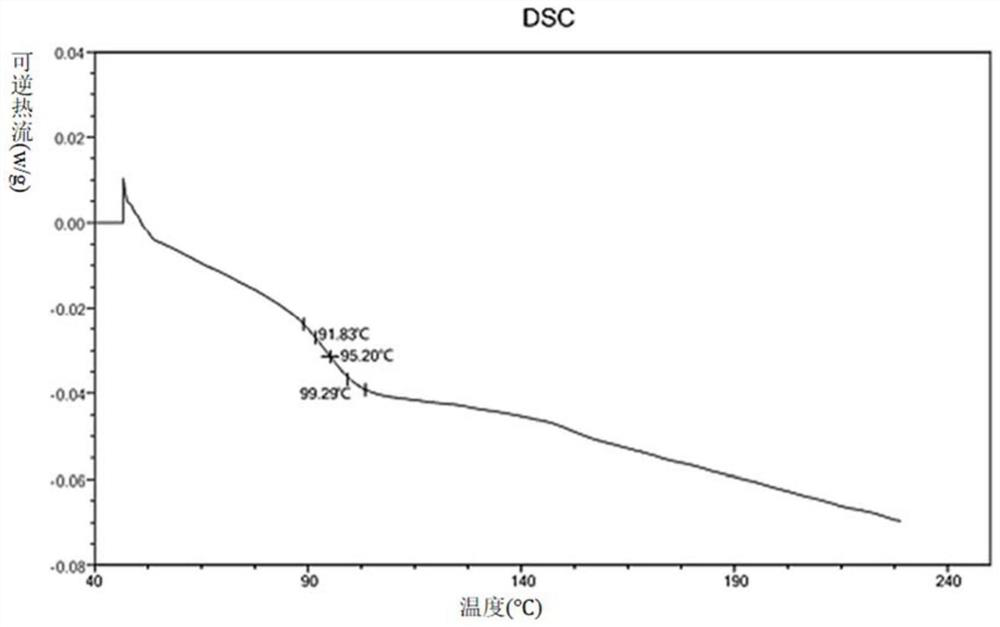
[0128] Amorphous or other crystalline forms of the compounds of formula (I) can also be used to prepare solid dispersions. Of course, it is also possible to prepare solid dispersions using salts of compounds of formula (I).
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com