Novel preparation containing anticoagulant drug cilostazol and preparation method thereof
A technology of cilostazol and pharmacy, which is applied to the new preparation of anticoagulant drug cilostazol and its preparation field, and can solve the problems of low dissolution rate, uneven dissolution rate of sustained-release preparations, and difficulty in control.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
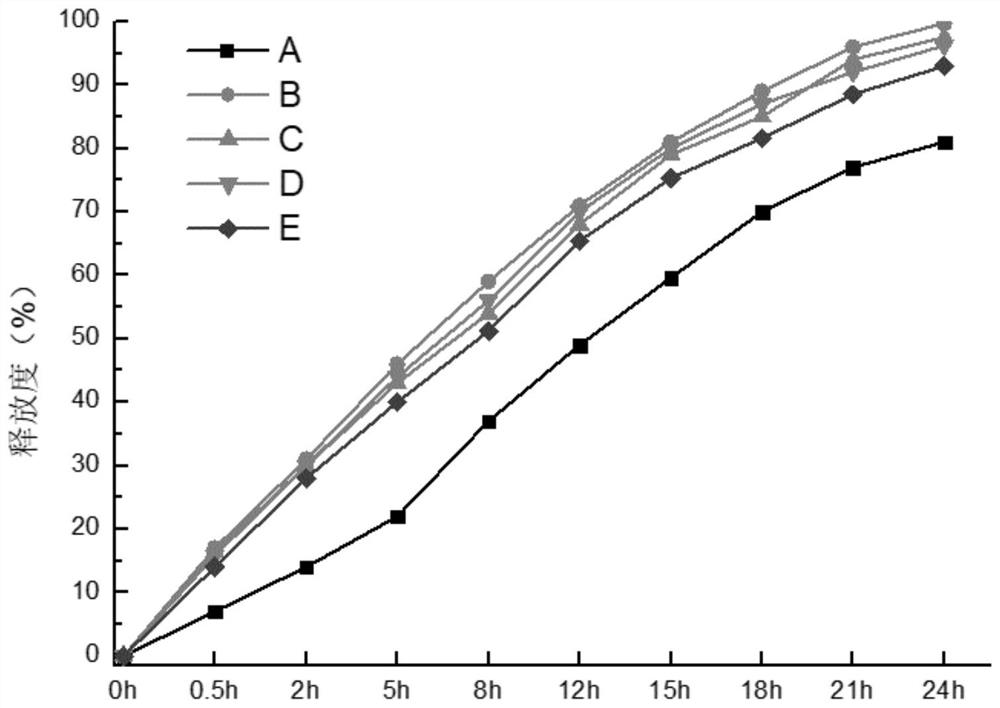
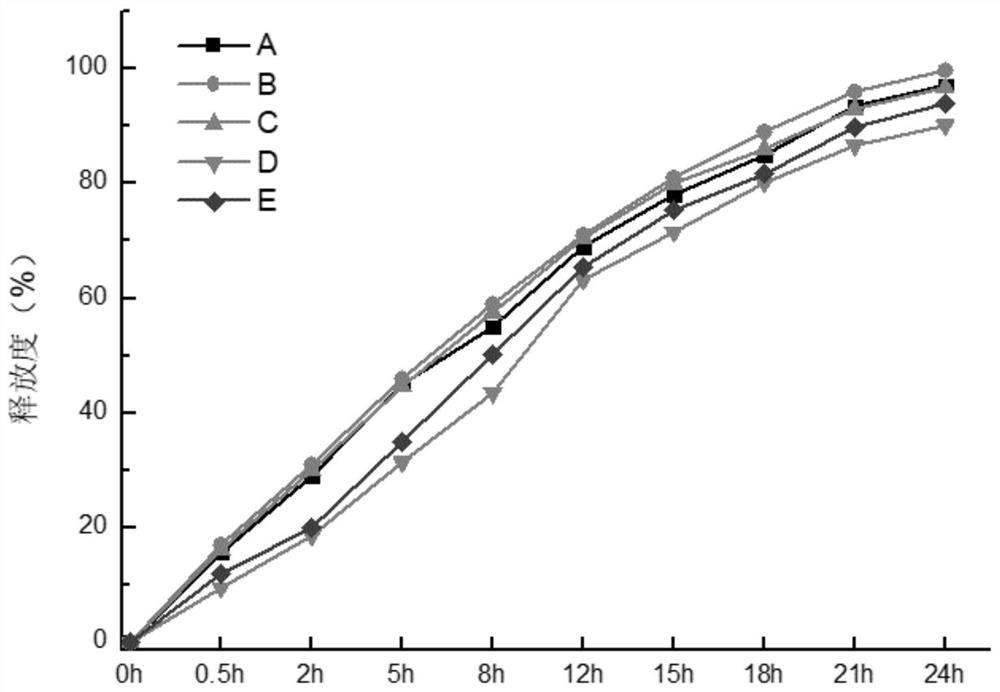
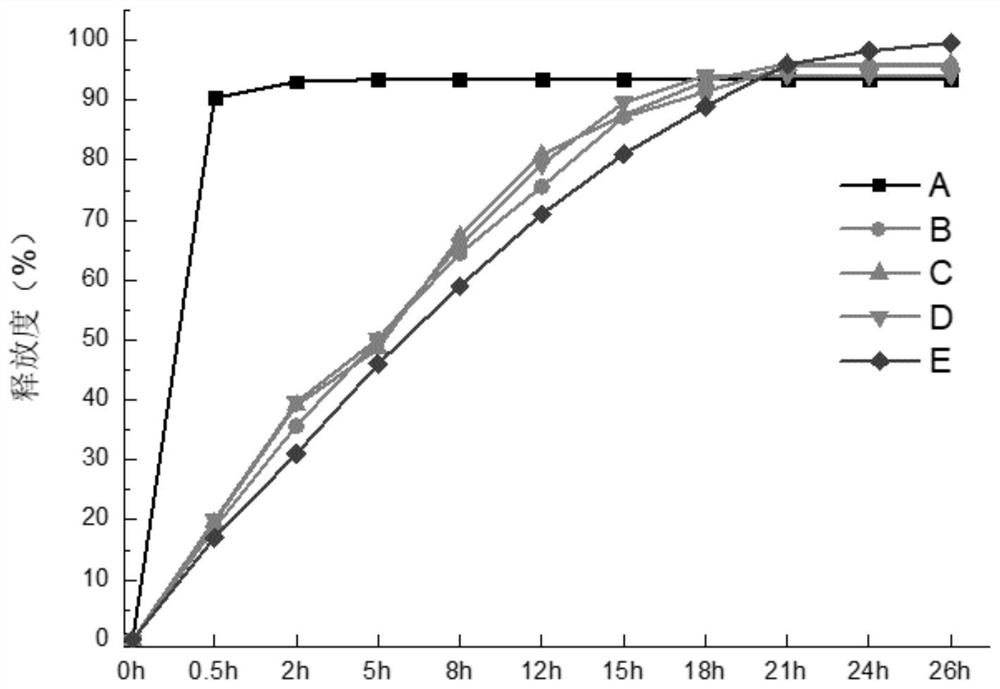
Image
Examples
Embodiment 1
[0036] Embodiment 1 Cilostazol double-layer sustained-release tablets (1000 tablets)
[0037] Immediate release layer:
[0038]
[0039]
[0040] Slow release layer:
[0041]
[0042] Preparation:
[0043] (1) Preparation of immediate-release layer: mix cilostazol and hydrophilic amino acid, pulverize together at low temperature and evenly, add the auxiliary materials in the pulverized formula, and mix to obtain the mixture powder of immediate-release layer;
[0044] (2) Preparation of sustained-release layer: Mix cilostazol with the auxiliary materials in the formula evenly, add an appropriate amount of purified water, and granulate to obtain the mixture of sustained-release layer particles;
[0045] (3) Granulation: The immediate-release layer mixture powder obtained in step (1) and the slow-release layer mixture granules obtained in step (2) are placed in a double-layer tableting machine and compressed into a double-layer tablet.
Embodiment 2
[0046] Embodiment 2 Cilostazol double-layer sustained-release tablets (1000 tablets)
[0047] Immediate release layer:
[0048]
[0049] Slow release layer:
[0050]
[0051] The preparation method is the same as in Example 1.
Embodiment 3
[0052] Embodiment 3 Cilostazol double-layer sustained-release tablets (1000 tablets)
[0053] Immediate release layer:
[0054]
[0055]
[0056] Slow release layer:
[0057]
[0058] The preparation method is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com