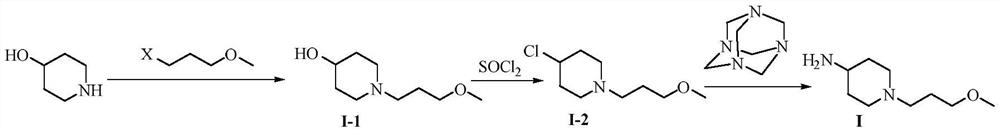
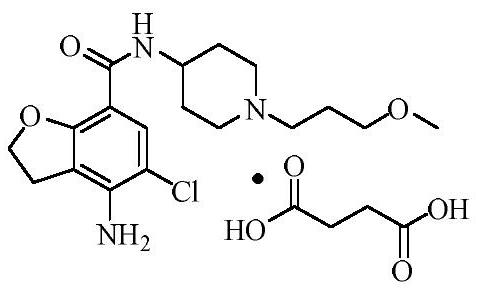
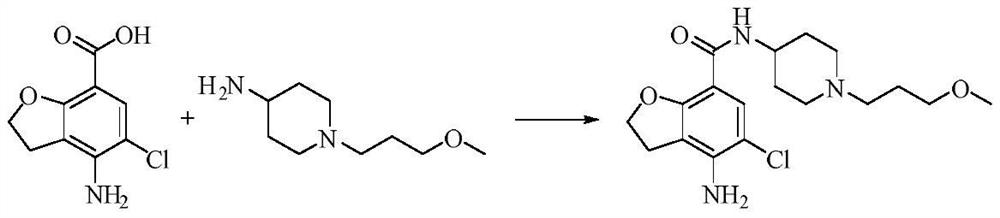
Synthetic method of prucalopride intermediate 1-(3-methoxy propyl)-4-piperidylamine
A technology of methoxypropyl and prucalopride, applied in the directions of organic chemistry, bulk chemical production, etc., to achieve the effects of avoiding operational danger, high yield and purity, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] 4-Hydroxypiperidine (40.46g, 0.40mol), 3-bromopropylmethyl ether (X=Br, 79.57g, 0.52mol), potassium carbonate (88.45g, 0.64mol) were added to acetonitrile (400ml), The temperature was controlled at 75 to 80 °C to react, and after the reaction was detected, filtered, the filtrate was concentrated to dryness under reduced pressure, dichloromethane (500 ml) was added, washed with 1M dilute hydrochloric acid (150 ml) for 1 to 2 times, the organic layer was dried, and the pressure was reduced. Concentrated to obtain intermediate I-1 with a yield of 96.4% and a purity of 99.3%.
Embodiment 2
[0078] 4-Hydroxypiperidine (40.45g, 0.40mol), 3-iodopropyl methyl ether (X=I, 88.01g, 0.44mol), triethylamine (64.76g, 0.64mol) were added to N,N-diethylamine In methylformamide (400ml), the temperature is controlled to react at 80-85°C. After detection, the reaction is completed, filtered, the filtrate is concentrated to dryness under reduced pressure, dichloromethane (500ml) is added, and 2M dilute hydrochloric acid (150ml) is used to wash 1~ twice, the organic layer was dried and concentrated under reduced pressure to obtain intermediate I-1 with a yield of 93.7% and a purity of 99.4%.
Embodiment 3
[0080] 4-Hydroxypiperidine (40.43g, 0.40mol), 3-chloropropyl methyl ether (X=Cl, 64.82g, 0.60mol), sodium bicarbonate (53.76g, 0.64mol) were added to butanone (400ml) In the reaction, the temperature was controlled at 75-80 °C, and after the reaction was detected, the filtrate was filtered, and the filtrate was concentrated to dryness under reduced pressure. Dichloromethane (500ml) was added, washed with 0.5M dilute hydrochloric acid (150ml) for 1 to 2 times, and the organic layer was dried. , concentrated under reduced pressure to obtain intermediate I-1 with a yield of 95.9% and a purity of 98.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com