Anti-B7-H3 monoclonal antibody, anti-B7-H3*CD3 bispecific antibody, preparation method and application of anti-B7-H3*CD3 bispecific antibody
A bispecific antibody, B7-H3 technology, applied in the fields of botanical equipment and methods, biochemical equipment and methods, antibodies, etc., can solve problems such as short half-life of antibodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0126] Example 1 Production of Anti-B7-H3 Monoclonal Antibodies and Bispecific Antibodies
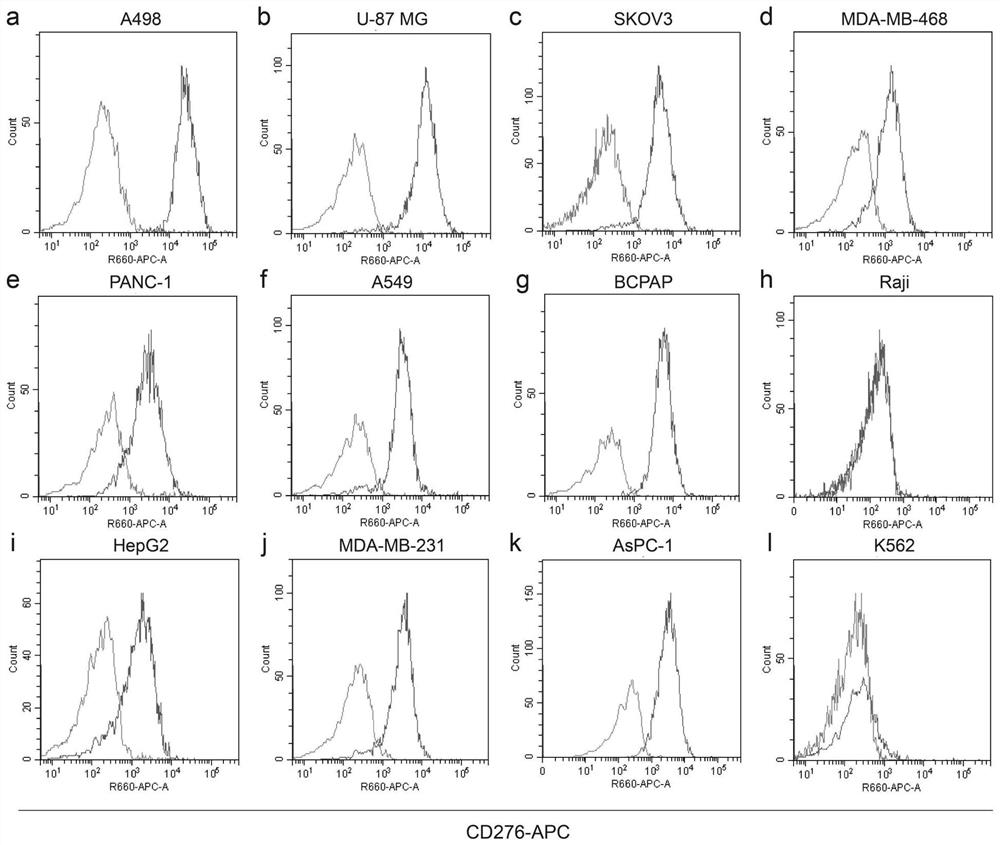
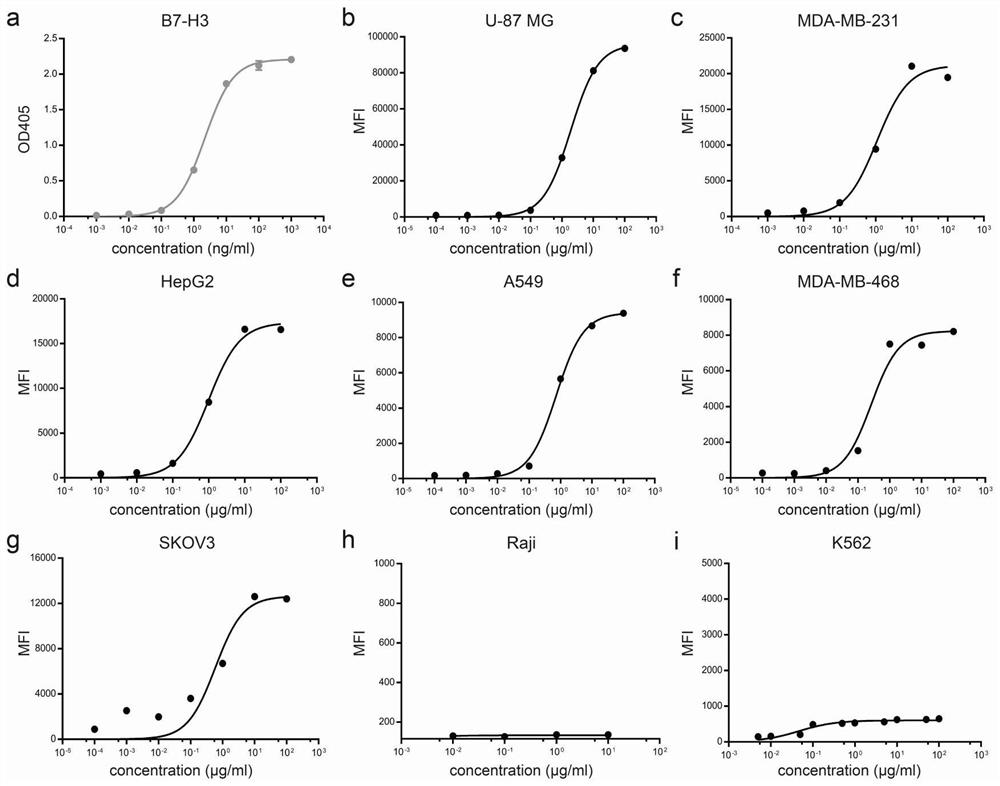
[0127] Bivalent anti-B7-H3 antibodies were prepared using hybridoma technology. BALB / c mice were immunized with recombinant human B7-H3-ECD protein (11188-H08H, SinoBiological) and screened for parental monoclonal antibodies against B7-H3 using ELISA for protein binding and flow cytometry for cell binding. Chimeric monoclonal antibody (10-2#c) is produced by fusing the light chain variable region (VL) sequence to the human Kappa constant region and the heavy chain variable region (VH) sequence to the human IgG1 constant region .
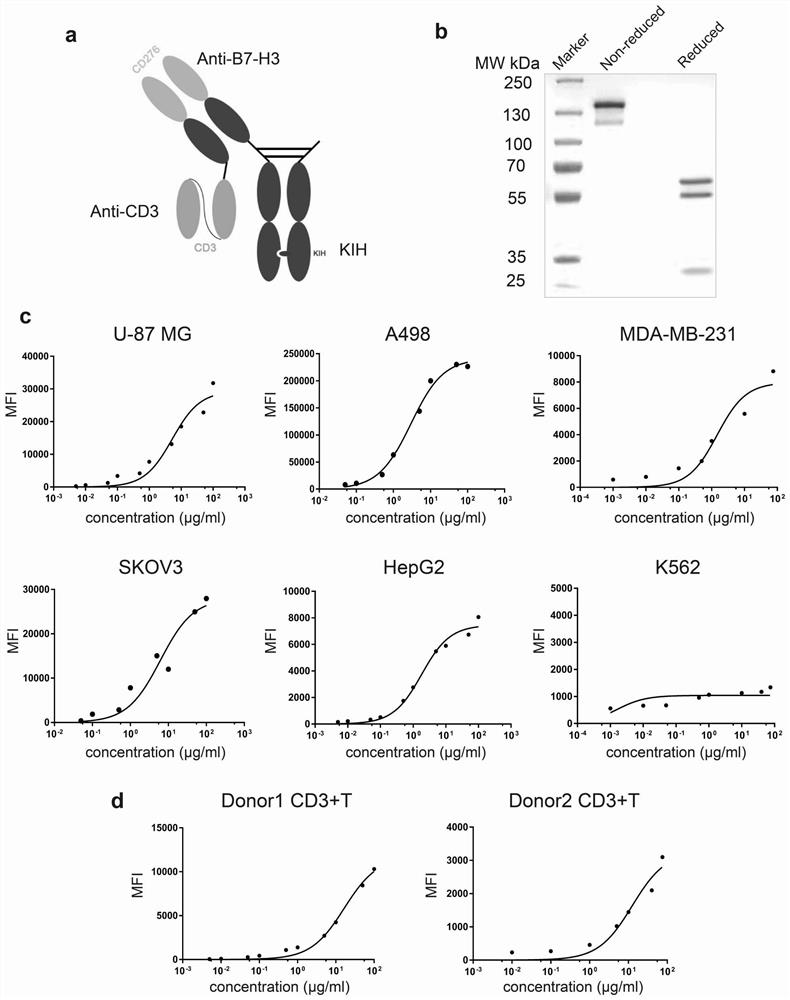
[0128] The anti-B7-H3×CD3 structure is a heterodimeric structure developed by knob-into-holes (KIH) technology and removes the effector function of IgG1 Fc by removing glycosylation in Fc (N297A). The Hole chain is the heavy chain of 10-2#c with mutations in the Fc region (N297A, T366S, L368A and Y407V). The knob chain is an IgGl Fc fragment with mutations ...
Embodiment 2
[0129] Example 2 Antibody expression and purification
[0130] The DNA fragments encoding the heavy and light chains were synthesized by Genewiz (Azenta Life Sciences) and cloned into the pcDNA3.1+ expression vector. Antibodies were expressed in FreeStyle™ 293T cells (Thermo Fisher Scientific) by PEI transfection. The supernatant was collected 7 days after transfection and the antibody was purified by protein A affinity chromatography (Thermo Fisher Scientific), after which the affinity chromatography product was further purified using anti-flag affinity gel (Biyuntian) (knob Fc fragment C-terminal with flag tag), protein samples were analyzed using SDS electrophoresis.
Embodiment 3
[0131] Example 3 Preparation of primary cells
[0132] Peripheral blood mononuclear cells (PBMCs) were isolated from the blood of healthy donors using Ficoll-Hypaque density gradient centrifugation (Tbdscience, Tianjin, China). PBMCs were cultured in IMDM medium (Hyclone) supplemented with 100 U / mL penicillin, 100 U / mL streptomycin and 10% heat-inactivated FBS. Human CD3+ T cells were isolated from PBMCs by negative selection using the Human CD3 T Cell Isolation Kit (480022; BioLegend) according to the manufacturer's protocol. Pre- and post-selection (positive and negative fractions) purity was tested using flow cytometry.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com