Preparation method of high-purity mepivacaine hydrochloride
A technology of mepivacaine hydrochloride and mepivacaine, which is applied in the field of biomedicine, can solve problems such as time-consuming and labor-consuming, impurity removal, loss of yield and high cost, and achieve the effect of simple operation and improved purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] A method for preparing high-purity mepivacaine hydrochloride, comprising the following steps:
[0048] S1, Preparation of mepivacaine crude products:
[0049] S11, 14kg of anhydrous formic acid was added to the 50-liter glass reactor, 3.8kgN- (2',6'-xylphenyl)-2-piperidinecarboxamide was added in batches at 20 °C, 50 minutes of heat preservation and stirring, 1.5kg of paraformaldehyde was added, heated to 95 °C, insulation reaction for 10 hours, after the reaction was completed, cooled to 25 °C, added 4N hydrochloric acid 9.95kg, and then reduced pressure to concentrate and remove the solvent;
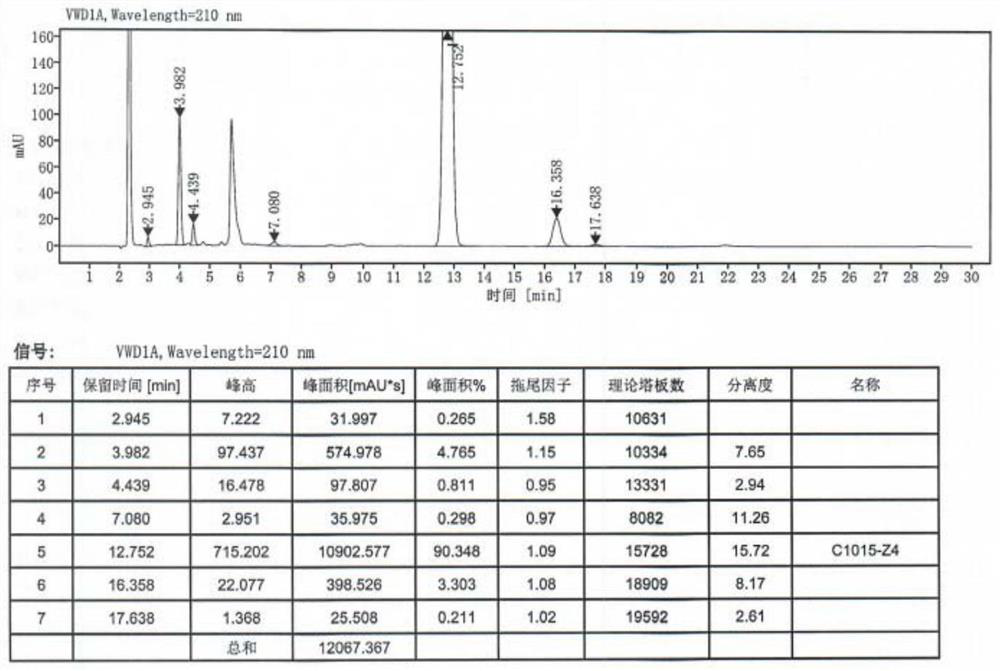
[0050] S12, remove the solvent and dissolve with 10kg of water stirring; Adjust pH ≥13 with 20% sodium hydroxide solution at 20 °C, filter, wash the filter cake with 2 kg of water, collect the filter cake, vacuum dry for 12 hours (vacuum degree is -0.08MPa, temperature is 55 °C), give crude mepivacaine 3.67kg, yield 91%, mp 148-152 °C; See high performance liquid chromatography Figu...
Embodiment 2
[0056] A method for preparing high-purity mepivacaine hydrochloride, comprising the following steps:
[0057] S1, Preparation of mepivacaine crude products:
[0058] S11, 280g of anhydrous formic acid was added to a 1-liter glass reactor, 76gN- (2',6'-xylphenyl)-2-piperidinecarboxamide was added in batches at 20 °C, stirred for 50 minutes, paraformaldehyde was added 30g, heated to 95 °C, insulation reaction for 10 hours, after the reaction was completed, cooled down to 25 °C, added 4N hydrochloric acid 200g, and then concentrated to remove the solvent under reduced pressure;
[0059] S12, remove the solvent with 200g of water stirred to dissolve; Adjust pH ≥13 with 20% sodium hydroxide solution at 20 °C, filter, wash the filter cake with 40 g of water, collect the filter cake, vacuum dry for 12 hours (vacuum degree -0.08MPa, temperature is 55 °C), to give mepivacaine crude product 73.6 g, yield 92%, mp 148-152 °C.
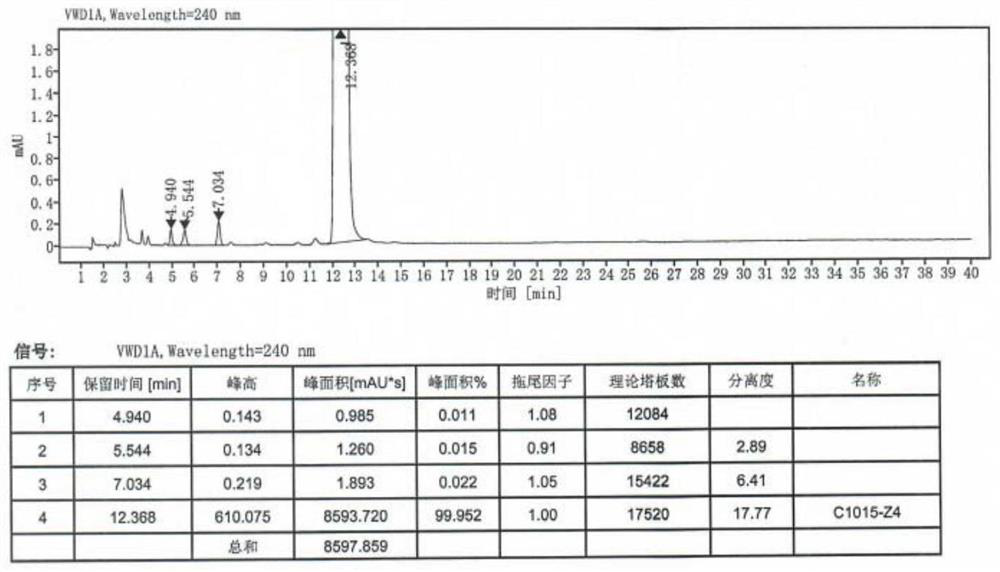
[0060] Preparation of S2, high-purity mepivacaine hydrochloride: ...
Embodiment 3
[0065] A method for preparing high-purity mepivacaine hydrochloride, comprising the following steps:
[0066] S1, Preparation of mepivacaine crude products:
[0067] S11, 1.4kg of anhydrous formic acid was added to a 5-liter glass reactor, 380gN- (2',6'-xylphenyl)-2-piperidinecarboxamide was added in batches at 20 °C, stirred for 50 minutes, added paraformaldehyde 150g, heated to 95 °C, insulation reaction for 10 hours, after the reaction, cooled down to 25 °C, added 4N hydrochloric acid 995g, and then reduced pressure to remove the concentrated solvent;
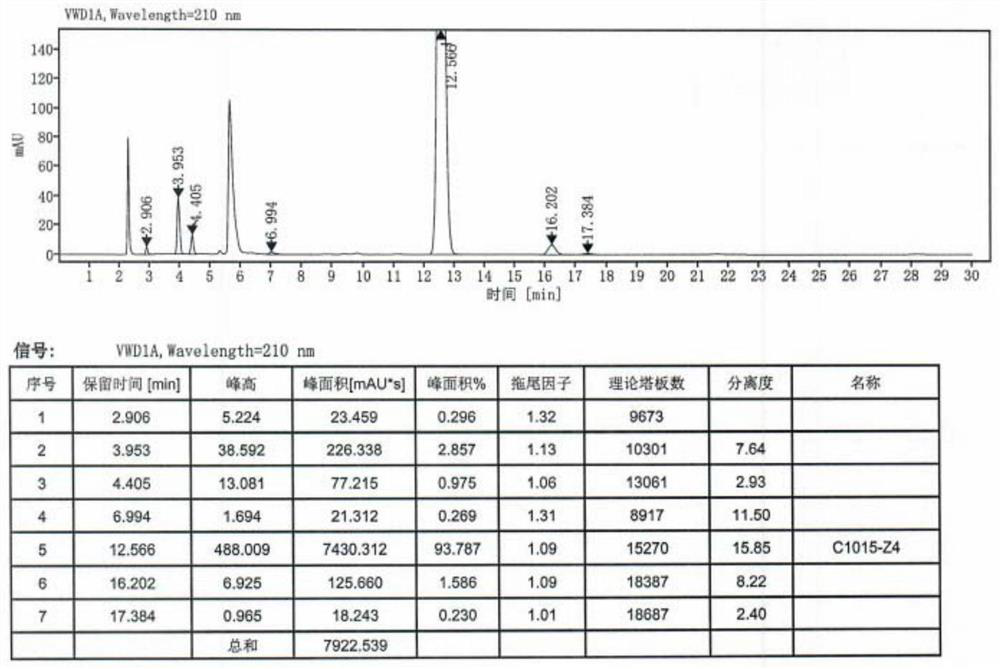
[0068] S12, remove the solvent and stir it to dissolve with 1kg of water; Adjust pH ≥13 with 20% sodium hydroxide solution at 20 °C, filter, wash the filter cake with 200g of water, collect the filter cake, vacuum dry for 12 hours (vacuum degree of -0.08MPa, temperature of 55 °C), to give mepivacaine crude product 0.372kg, yield 91.8%, mp 148-152 °C.
[0069]Preparation of S2, high-purity mepivacaine hydrochloride:
[0070] S21、...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com