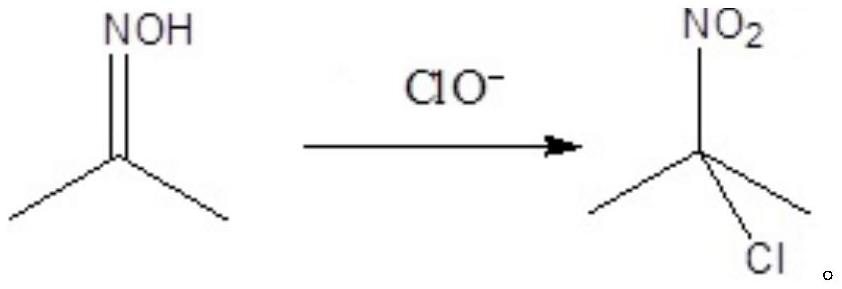
Preparation method of 2-chloro-2-nitropropane
A technology of nitropropane and acetone oxime, which is applied in the field of preparation of 2-chloro-2-nitropropane, can solve the problem of low reaction yield and achieve the effect of reducing the cost of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
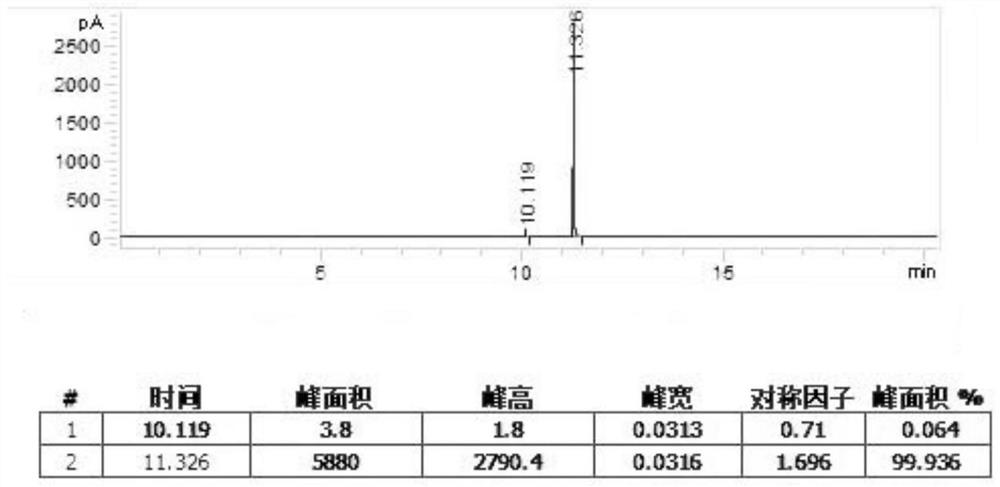
[0044] In a 5L three-necked bottle equipped with an electric stirrer, a thermometer and a solid feeding port, 3736.17 g of sodium hypochlorite solution was added at room temperature, and the available chlorine content was 13.0%. Under the conditions of stirring and temperature control at 15°C, 250 g of acetone oxime was added to the three-necked flask in batches, and the addition time was 1.0 h. After the addition was completed, the reaction was continued to stir at room temperature for 0.5 h. After the reaction was completed, the reaction solution was placed in a separatory funnel and left to stand for stratification, and the separated lower layer liquid was 361.29 g of 2-chloro-2-nitropropane product, with a yield of 85.5%. GC detection, purity 99.6%.
Embodiment 2
[0046] In a 5L three-necked flask equipped with an electric stirrer, a thermometer and a solid feeding port, 1810.61 g of sodium hypochlorite solid (content 90%) and 1922.61 g of water were added successively at room temperature. Under the conditions of stirring and temperature control at 15°C, a total of 400 g of acetone oxime was added to the three-necked flask in batches, and the addition time was 1.5 h. After the addition was completed, the reaction was continued to stir at room temperature for 0.5 h. After the reaction was completed, the reaction solution was placed in a separatory funnel to stand for stratification, and the lower layer liquid was separated to obtain 561.16 g of the 2-chloro-2-nitropropane product, with a yield of 83.0%. GC detection, purity 99.5%.
[0047] 2-Chloro-2-nitropropane product yield is mass yield: measure the moisture of the product separated from the separatory funnel by Karl Fischer's moisture measurement method to obtain the purity of the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com