4-(dihydroxyacetyl-L-serine)-goserelin as well as preparation method and application thereof
A technology of goserelin and preparation, applied in the field of medicine, can solve problems such as lack of research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
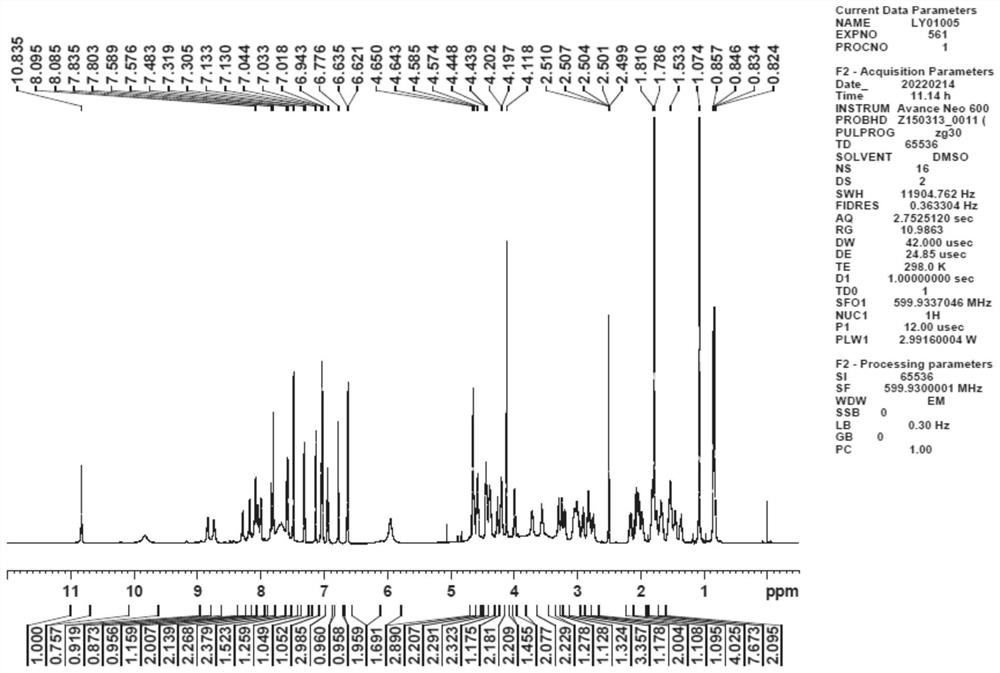
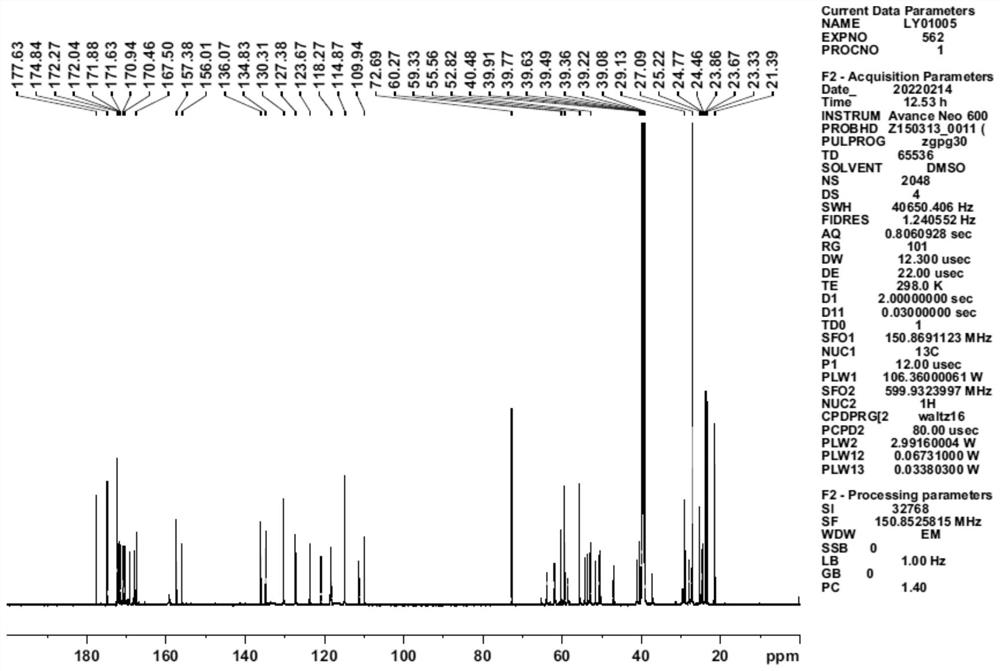
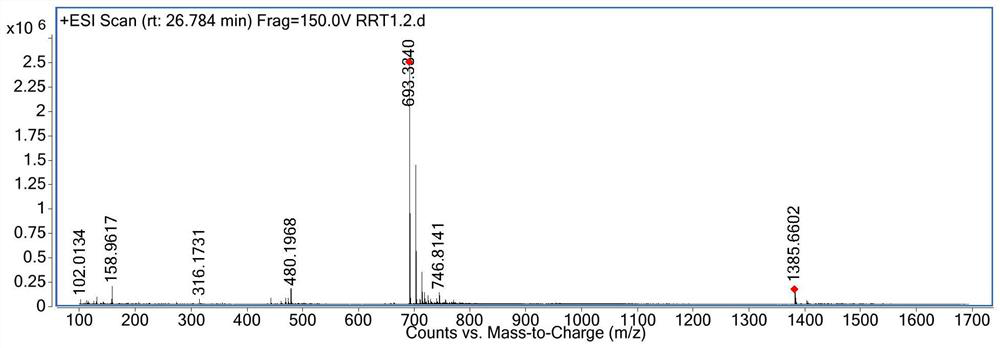
[0036] Example 1 Preparation of 4-(diglycolyl-L-serine)-goserelin
[0037] Step 1: Add 500 mg of goserelin, 50 mg of glycolide, and 5 ml of DMF solvent to a 10 ml vial, and react at room temperature for 24 hours.
[0038] Step 2: The reaction solution in step 1 was separated by preparative high performance liquid chromatography (chromatographic column: phenomenonex, luna 5 μm, 250×21.20mm, S / N: 443257-2, flow rate 20ml / min, detection wavelength 220nm), at 0.5 % Phosphoric acid aqueous solution-acetonitrile was used as the mobile phase, the ratio of acetonitrile was 20%-40% for gradient elution, the chromatographic peak was collected with a retention time of 14.03min, and a total of 750ml of eluent was collected.
[0039] Step 3: Mix the reversed-phase silica gel with methanol, stir, homogenize and pack the column, replace the methanol with purified water, add the eluent obtained in step 2 to the reversed-phase silica gel chromatography column with a peristaltic pump, adjust th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com