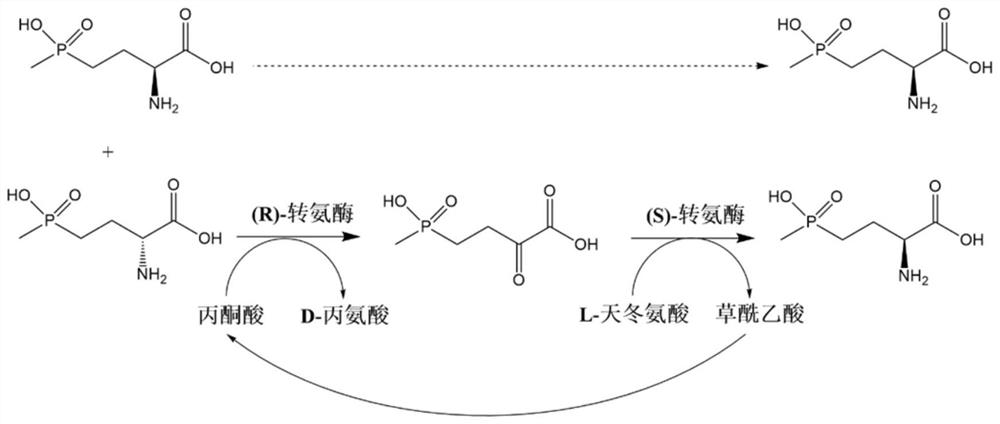
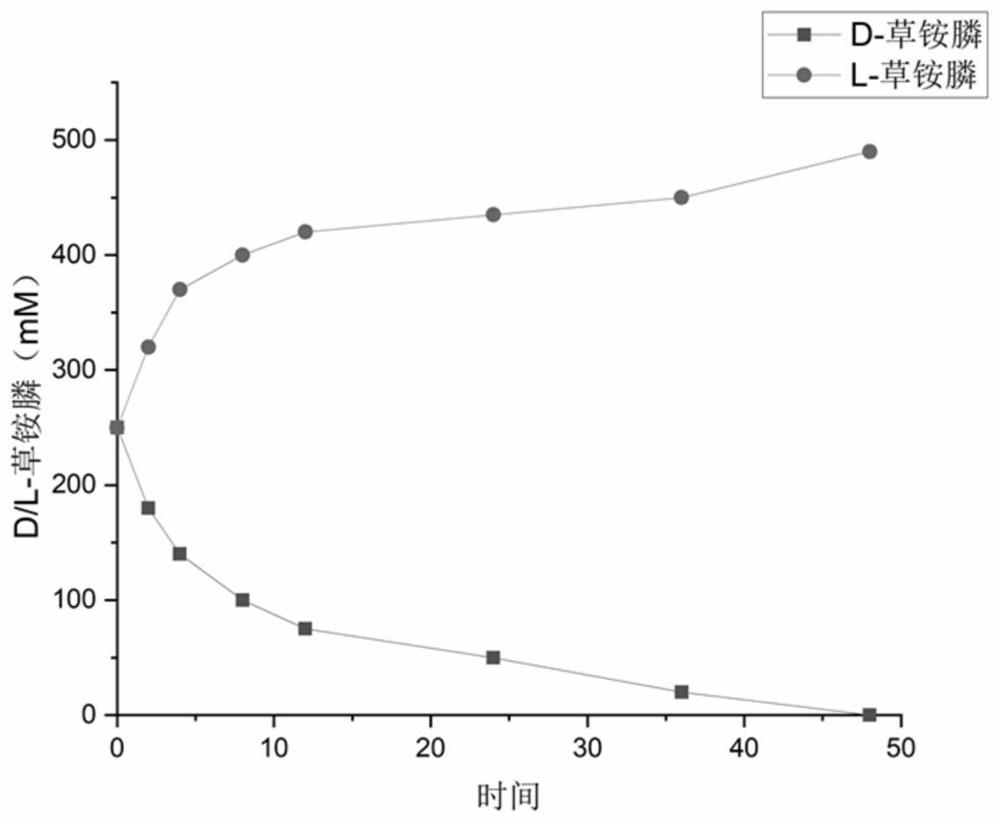
(S)-transaminase and application thereof
A transaminase and amino technology, which is applied in the field of preparing L-glufosinate-ammonium by biological multi-enzyme coupling method, can solve the problems of difficulty in product separation and purification, difficulty in increasing the amount of substrate input, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1: Cultivation of engineered bacteria cells
[0050] The engineered bacteria E.coli BL21(DE3) / pCDFduet-1-APH1, E.coli BL21(DE3) / pCDFduet-1-EN5, E.coli BL21(DE3) / pCDFduet-1-APH1-EN5 were streaked on a plate After activation, single colonies were inoculated into 10 mL of LB liquid medium containing 50 μg / mL kanamycin, and incubated at 37°C with shaking for 10 h. Transfer to 50 mL of LB liquid medium containing 50 μg / mL kanamycin at 2% of the inoculum, and shake at 37 °C until the OD600 reaches about 0.8, add IPTG with a final concentration of 0.5 mM, and shake at 28 °C Incubate for 12h. After the cultivation, the culture solution was centrifuged at 8000 rpm for 10 min, the supernatant was discarded, the cells were collected, and stored in a -80°C ultra-low temperature refrigerator until use.
Embodiment 2
[0051] Example 2: Enzyme sequence synthesis and strain construction
[0052] The sequence derived from Pseudarthrobacter chlorophenolicus annotated as (R)-transaminase (APH1) (the amino acid sequence is shown in SEQ ID NO. The plasmid pET-28a(+) was expressed to give pET28a-APH1. After sequencing and verification, the pET28a-APH1 was transferred into the expression host E. coli BL21 (DE3) for subsequent expression of the recombinase.
[0053] The sequence derived from Corynebacterium vitaeruminis DSM 20294 annotated as (S)-transaminase (EN5) (the amino acid sequence is shown in SEQ ID NO.3, the nucleotide sequence is shown in SEQ ID NO.4) was subjected to full gene synthesis, Insert into expression plasmid pET-28a(+) to obtain pET28a-EN5. After sequencing and verification, the pET28a-EN3 was transferred into the expression host E. coli BL21 (DE3) for subsequent expression of the recombinase.
Embodiment 3
[0054] Example 3: Construction of co-expression strains containing (R)-transaminase and (S)-transaminase systems:
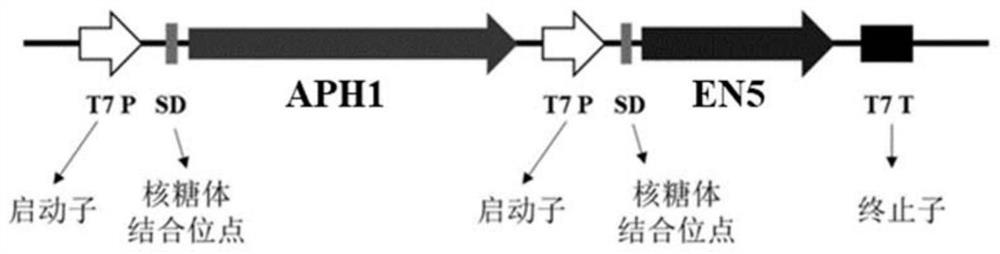
[0055] The APH1 gene used in Example 2 was connected to the multi-cloning site vector pCDFduet-1 by a one-step cloning kit, the restriction sites were HindIII and XhoI, and the one-step cloning primers were C1-F and C1-R (Table 1) , the plasmid pCDFduet-1-APH1 was constructed. On the basis of the pCDFduet-1-APH1 plasmid, the EN5 used in Example 2 was connected to the second cloning site of the multi-cloning site vector pCDFduet-1 by a one-step cloning kit, and the restriction sites were NdeI and XhoI, the one-step cloning primers were C2-F and C2-R, the plasmid pCDFduet-1-APH1-EN5 was constructed, and the co-expression strain E.coli BL21(DE3) / pCDFduet-1-APH1-EN5 was constructed. APH1-EN5 constructs such as figure 2 shown.
[0056] Table 1: Cloning primer sequences
[0057] primer sequence C1-F CCCAAGCTTAAGGAGATATACATATGACCTCTCCCGCTTCCGT ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com