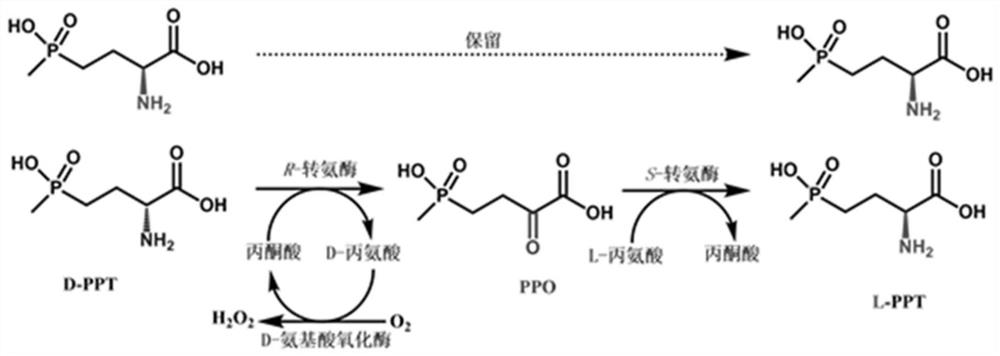
Method for preparing L-glufosinate-ammonium by using biological multi-enzyme coupling method
A technology of glufosinate-ammonium and product, which is applied in the field of preparing L-glufosinate-ammonium by biological multi-enzyme coupling method, can solve problems such as difficulty in product separation and purification, difficulty in increasing substrate dosage, etc., and achieve high product yield and production The effect of low cost and high conversion rate of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Embodiment 1: the cultivation of engineering bacterium thalline
[0083] The engineered bacteria were recombinant Escherichia coli E. coli BL21(DE3) / pET-28a-APH1, E.coli BL21(DE3) / pET-28a-DAAO, E.coli BL21(DE3) / pET-28a-EN3, E.coli BL21(DE3) / pET-28a-EN3, E. After coli BL21(DE3) / pET-28a-PDC was activated by streaking on a plate, a single colony was picked and inoculated into 10 mL LB liquid medium containing 50 μg / mL kanamycin, and cultured with shaking at 37°C for 10 h. Transfer 2% of the inoculum into 50 mL of LB liquid medium also containing 50 μg / mL kanamycin, culture with shaking at 37 °C until the OD600 reaches about 0.8, add IPTG with a final concentration of 0.5 mM, and shake at 28 °C Cultivate for 12h. After the cultivation, the culture solution was centrifuged at 8000rpm for 10min, the supernatant was discarded, the bacteria were collected, and stored in a -80°C ultra-low temperature refrigerator until use.
Embodiment 2
[0084] Embodiment 2: Enzyme sequence synthesis and bacterial strain construction
[0085] After the sequence (R)-transaminase (APH1) annotated from Pseudarthrobacter chlorophenolicus (the amino acid sequence is shown in SEQ ID NO.1, the nucleotide sequence is shown in SEQ ID NO.2) after the whole gene synthesis, insert The expression plasmid pET-28a(+) was used to obtain pET28a-APH1. After sequencing verification, pET28a-APH1 was transferred into the expression host E. coli BL21 (DE3) for subsequent expression of the recombinase.
[0086] The sequence annotated as D-amino acid oxidase (DAAO) derived from Rhodotorula sp.CCFEE 5036 (the amino acid sequence is shown in SEQ ID NO.3, and the nucleotide sequence is shown in SEQ ID NO.4) was carried out for whole gene synthesis Finally, the expression plasmid pET-28a(+) was inserted to obtain pET28a-DAAO. After sequencing verification, pET28a-DAAO was transferred into the expression host E. coli BL21 (DE3) for the subsequent expres...
Embodiment 3
[0089] Example 3: Resolution of racemic PPT with recombinant Escherichia coli E.coli BL21(DE3) / pET-28a-APH1 and E.coli BL21(DE3) / pET-28a-DAAO
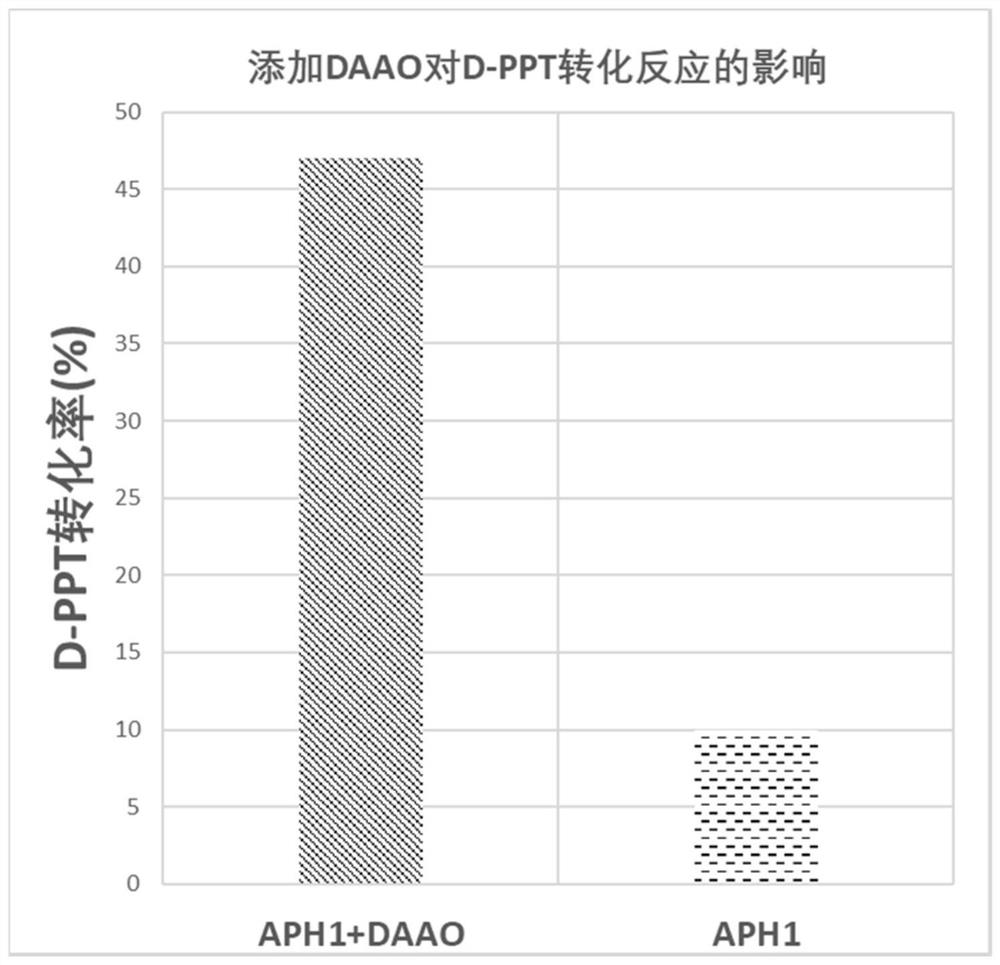
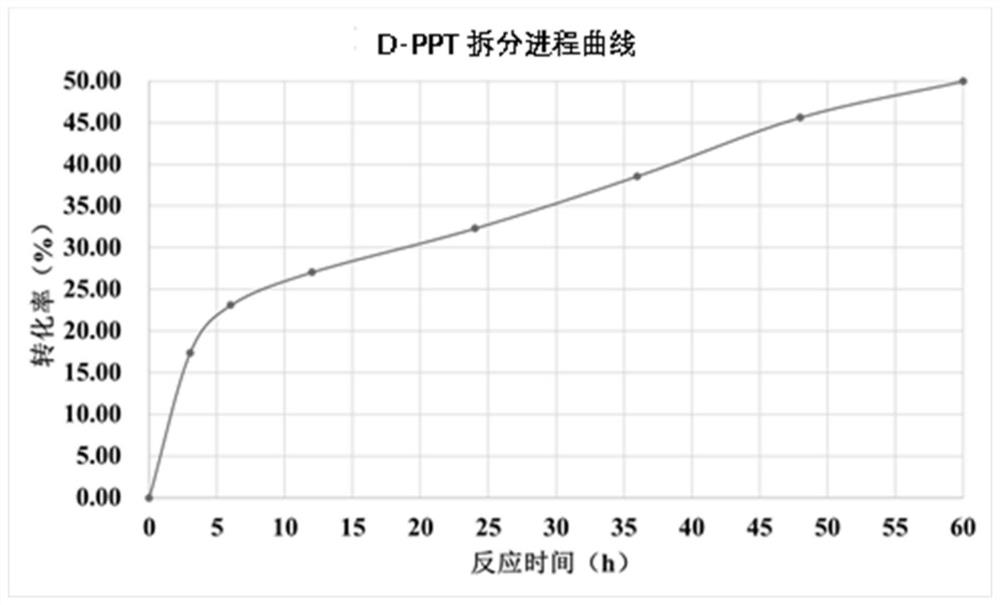
[0090] (i) Recombinant Escherichia coli E.coli BL21(DE3) / pET-28a-APH1 single-enzyme catalytic system experiment: 30ml reaction system contains 500mM D,L-PPT, 250mM pyruvate, and 100mM phosphate buffer, use Ammonia water was used to adjust the pH of the reaction system to 8.0, and 30 g / L stem cells of recombinant E. coli E. coli BL21(DE3) / pET-28a-APH1 were added. The reaction conditions are: temperature 30° C., rotation speed 250 rpm. After 10 h of reaction, samples (100 μl) were taken, 900 μl of deionized water was added, and the reaction was terminated by heating. Detect the conversion situation of D-PPT by HPLC, the reaction result is as follows diagram 2-1 shown.
[0091] (ii) Recombinant Escherichia coli E.coli BL21(DE3) / pET-28a-APH1 and E.coli BL21(DE3) / pET-28a-DAAO dual enzyme catalytic system experiment: 30ml reaction system...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com