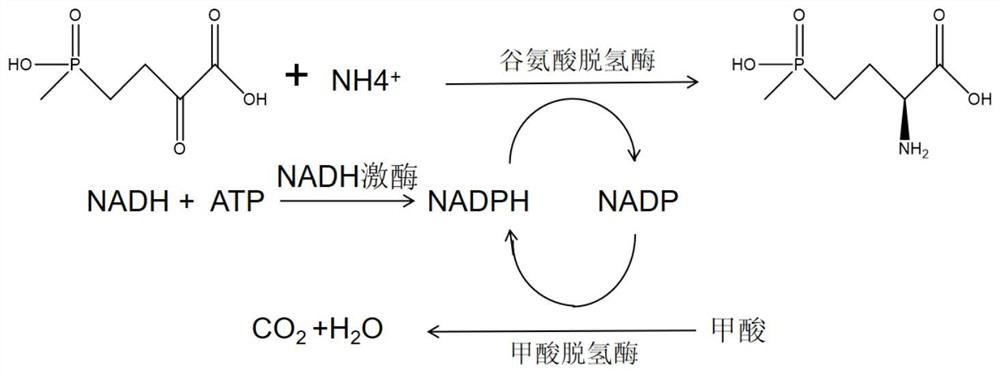
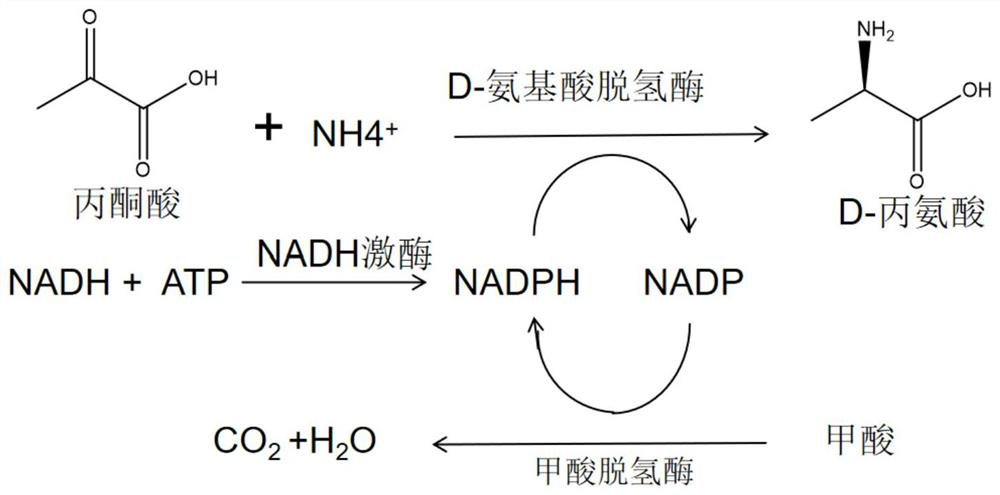
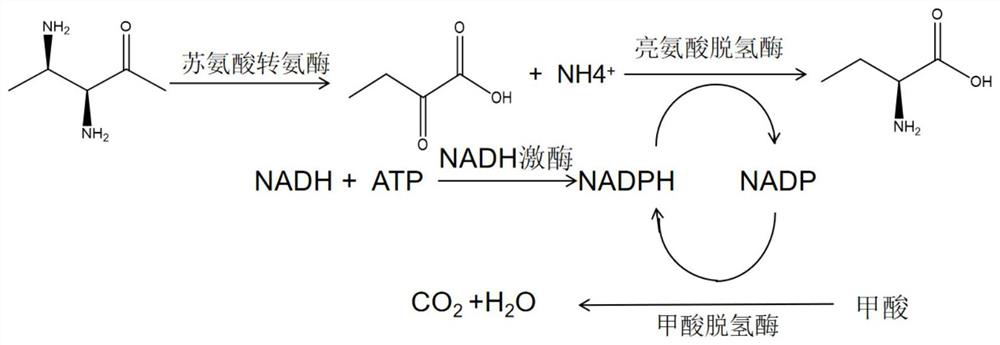
NADH kinase mutant, coding gene and application
A technology of mutants and kinases, applied in the field of NADH kinase mutants and their coding genes, can solve the problems of unavailable, expensive, and reduced utilization of raw materials, and achieve the effect of reducing the amount of use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: Construction of NADH kinase genetically engineered bacteria
[0041] The gene sequence of NADH kinase (Gen Bank No.: NC_001148.4) from Saccharomyces cerevisiae was codon-optimized and sent to Sangon Bioengineering (Shanghai) Co., Ltd. for whole gene synthesis, and cloned into the recombinant expression plasmid pET-28a (+) on. The codon-optimized NADH kinase gene sequence is shown in SEQ ID No.2.
[0042] Since the expression vector used for glutamate dehydrogenase and formate dehydrogenase is the pETDuet-1 plasmid, which cannot coexist with the pET-28a(+) plasmid, the recombinant expression vector of NADH kinase was replaced with the pCDFDuet plasmid. The empty pCDFDuet plasmid was used as a template, and pCDFDuet-Pf and pCDFDuet-Pr in Table 1 were used as primers for PCR. This process was called plasmid linearization. Using the synthetic recombinant plasmid pET-28a(+) as a template, NADHK-Pf and NADHK-Pr in :1 are used as primers for amplification, and ...
Embodiment 2
[0046] Embodiment 2: Construction of NADH kinase mutant library
[0047] In the first round, the above-mentioned preserved bacterial solution was cultured in a test tube, and the plasmid was extracted as a template for site-directed mutation PCR of NADH kinase mutation, and T175H-Pf and T175H-Pr in Table 2 were used as mutation primers to perform site-directed mutation PCR. The PCR product was transformed, plated, and the T175H mutant was obtained by screening, which was named T175H.
[0048] In the second round, site-directed mutagenesis PCR was performed using T175H as a template and R252A-Pf and R252A-Pr in Table 2 as primers. The PCR product was transformed, plated, and the mutant of T175H mutation and R252A was obtained by screening, which was named as T175H-R252A.
[0049] In the third round, T175H-R252A was used as a template, and I332K-Pf and I332K-Pr in Table 2 were used as primers for site-directed mutagenesis PCR. The PCR product was transformed, plated, and the T...
Embodiment 3
[0055] Embodiment 3: the construction of double plasmid engineering bacterium
[0056] The glutamic acid dehydrogenase gene and the formate dehydrogenase gene are respectively located at the first multiple cloning site and the second multiple cloning site on the plasmid PetduetI, and the plasmid is named PetduetI-GluDH-FDH. The NADH kinase gene is located in a multiple cloning site of the PcdfduetI plasmid, named PcdfduetI-NADHK. The two plasmids were extracted using a plasmid extraction kit, introduced into the same BL21 competent cell, spread on a plate containing both streptomycin and ampicillin resistance, and cultured for 12 hours. Pick a single colony and inoculate it into 10ml LB liquid medium containing 50ul / ml streptomycin and 50ul / ml ampicillin resistance, and culture with shaking at 37°C for 10h. Add 30% glycerol to the bacterial solution at a ratio of 1:1, and store in a -80°C refrigerator.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com