Recombinant coxsackie B3 virus with fluorescent protein tag and construction method
A fluorescent protein and construction method technology, applied in the field of recombinant Coxsackie B3 virus and construction, can solve the problem of insufficient and in-depth analysis of the dynamic process of the virus, and achieve the effect of a stable and reliable research and application platform
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
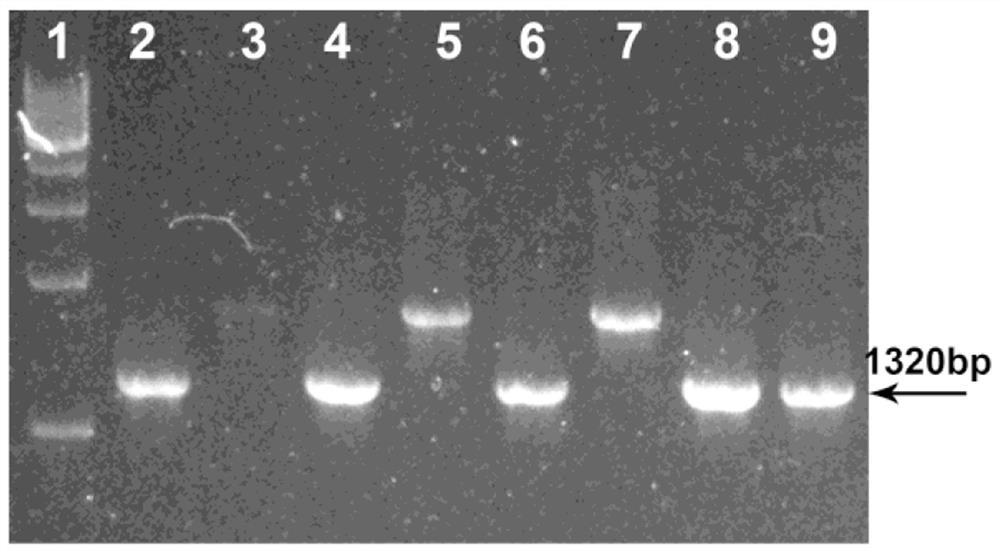
[0036] In order to facilitate the introduction of foreign genes into the CVB3 genome, on the basis of the constructed full-length infectious clone (pCV) of CVB3, this experiment introduced the Multiple Cloning Site (MCS) into the P1 region of the CVB3 genome and P2 region, and the restriction recognition sequence of 2Apro (TTMTNTG / AFGQQSGA) was added before the multiple cloning site. According to the sequencing sequence of pCV, DNASTAR Lasergene 9.0 software was used to design 2 pairs of primers for cloning insertion of MCS and 2Apro restriction recognition sequence with a length of 42bp (Table 1-1). In order to facilitate the integration of the full-length gene into the plasmid, the XhoI restriction site was introduced into the upstream sequence of the first pair of primers, and the SpeI restriction site was designed in the downstream primer sequence of the second pair of primers. The primers used were synthesized by Shenzhen Huada Gene Company.
[0037] Table 2-1 Constructi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com