Immune-enhanced EV71 vaccine soluble microneedle
A technology of EV71 and immune enhancement, which is applied in the fields of pharmaceutical devices, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the difficulties, uncertainties, and differences in the mechanical properties of soluble microneedles, etc. problem, achieve the effect of enhancing immunogenicity and good mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1EV71
[0019] Example 1 Preparation of EV71 vaccine soluble microneedles
[0020] The EV71 vaccine soluble microneedle to be developed in the present invention is composed of EV71 vaccine, adjuvant and matrix material, etc. The preparation method is as follows:
[0021] 1) Preparation of microneedle male mold: The shape, height, spacing of the male mold mold and the density of the microneedle array are designed by computer aided design, and stainless steel is selected as the processing material to be processed and formed according to the design size.
[0022] 2) Preparation of the microneedle female mold: after mixing the polysiloxane and the curing agent in a mass ratio of 10:1, pour it into a cuboid container with a single crystal silicon male mold microneedle; Vacuum was evacuated for 15 minutes at a temperature of 0.1 MPa to remove air bubbles in the mixed solution; it was then placed in an oven, dried at 70° C. for 3 hours, and then taken out to obtain a molded polysiloxane nega...
Embodiment 2
[0025] Example 2 Effects of different types of adjuvants on the mechanical properties of EV71 vaccine soluble microneedles
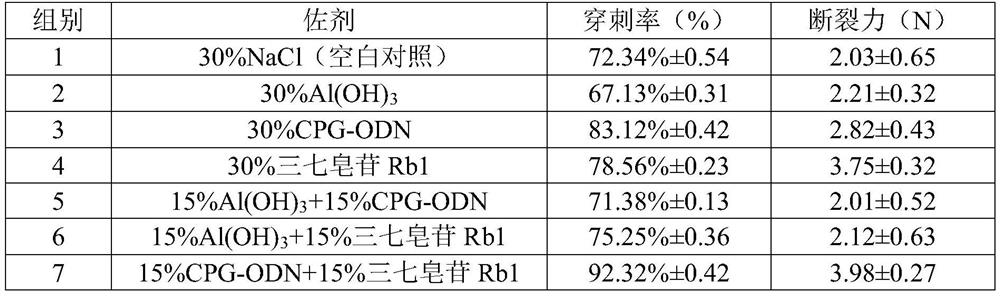
[0026] In order to investigate the influence of different types of adjuvants on the soluble microneedles of EV71 vaccine, this example assumes that the mass proportion of adjuvant in the needle body is a certain value (30%), only the type of adjuvant is changed, and the adjuvant is aluminum hydroxide. , CPG-ODN, Panax notoginseng saponins Rb1 or their combination to compare the immune effect.
[0027] In this example, the prescription and preparation method of EV71 vaccine soluble microneedle refer to Example 1, and the measurement method of mechanical properties is as follows:
[0028] Method 1): Using a physical property analyzer to investigate the pressure change performance of microneedles
[0029]Place the soluble microneedle with the needle face up, so that the direction of the needle is parallel to the axial direction of the probe, measure the pr...
Embodiment 3
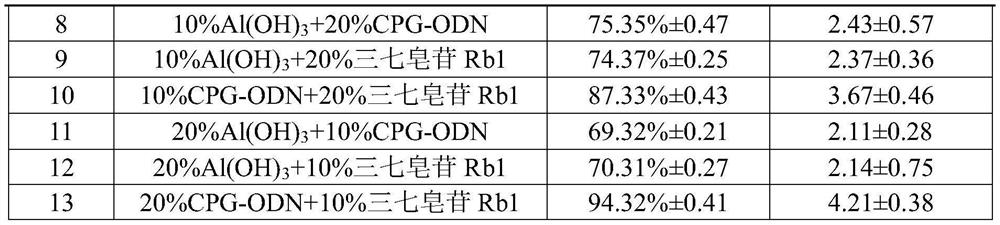
[0041] Example 3 The effect of the change of the mass ratio of each component in the adjuvant on the immune effect of EV71 soluble microneedle
[0042] It can be seen from Example 2 that the best adjuvant selection is the combination of CPG-ODN and Panax notoginseng saponin Rb1. In this experiment, this compound adjuvant will be screened by the mass ratio of each component, and the difference in the immune effect of microneedles will be further investigated. Filter out the best mass ratio.
[0043] Micro-needle prescription and preparation reference example 2, the evaluation method of immune effect is as follows:
[0044] The experimental animals were selected 6-8 weeks old Balb / c female mice, and the back of the mice was depilated, and then administered with microneedles on the back. 14 days after immunization, the peripheral blood of the mice was detected by a cytokine kit. The content of cytokines IL-6, IL-10 and IFN-γ, wherein the method is to collect the spleen from the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Breaking force | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com