Antibody for resisting FGF21 carboxyl terminal and application thereof
A FGF21, carboxy-terminal technology, applied in the fields of application, anti-animal/human immunoglobulin, genetic engineering, etc., to achieve the effect of clear antigen binding sites
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1 Animal immunization
[0042] Four polypeptides were designed and synthesized, three of which contained three amino acids (YAS) at the carboxyl terminus of FGF21, and one did not contain YAS. The sequences are shown in Table 1.
[0043] Table 1 Synthetic polypeptide sequences
[0044]
[0045] The polypeptide A for immunization was coupled separately for animal immunization.
[0046] After three immunizations, blood was collected to measure serum titers. The experimental groups of immunized animals are shown in Table 2.
[0047] Table 2 Information on immunized animals
[0048]
[0049]
[0050] Using the indirect enzyme-linked immunoassay method (indirect ELISA), the animal serum with high titer for immunization polypeptide A and low titer for screening polypeptide B was screened. The specific steps include: coating the ELISA plate with polypeptide A for immunization, polypeptide B for screening (SinoA8627), protein A (SEQ ID NO: 22) and protein B...
Embodiment 2
[0059] Example 2 Fusion hybridoma cells and screening
[0060] According to the results in Table 3-5, the mouse numbered SBI180064-2#C was selected to be injected with the polypeptide SinoA8624 for booster immunization; 3 days later, the spleen was taken for fusion, and the fused cells were suspended in HAT medium, according to the ratio of 6 × 10 per well. 4 Each cell was seeded in a 96-well cell culture plate, that is, a total of 30 96-well cell culture plates were seeded. On the 6th day and the 8th day after fusion, the medium in the culture plate was discarded, fresh HAT medium was added, and the hybridoma cell culture supernatant was taken on the 10th day after fusion for primary clone screening (the results are shown in Table 6). ).
[0061] Table 6 ELISA test results of primary clone screening for the first time
[0062]
[0063] The positive cells obtained by screening were counted, and then seeded into a 96-well cell culture plate at 0.75 cells / well, and 0.5 cell...
Embodiment 3
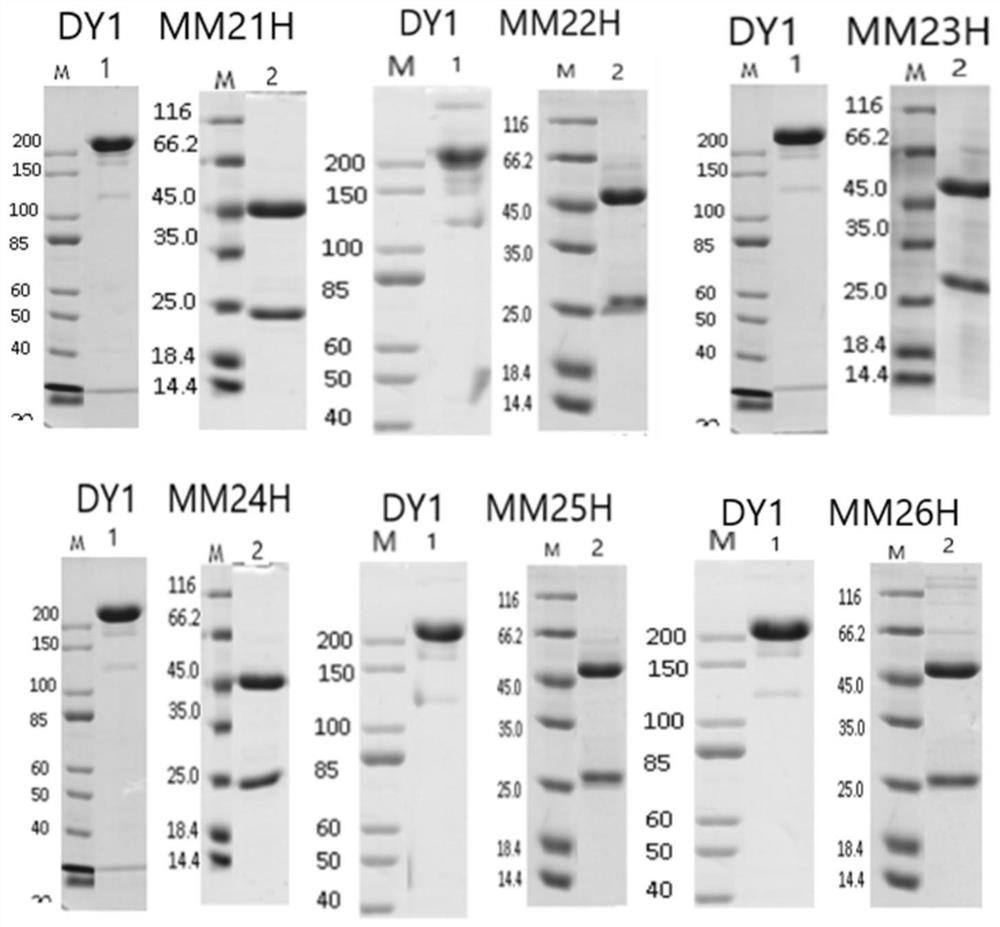
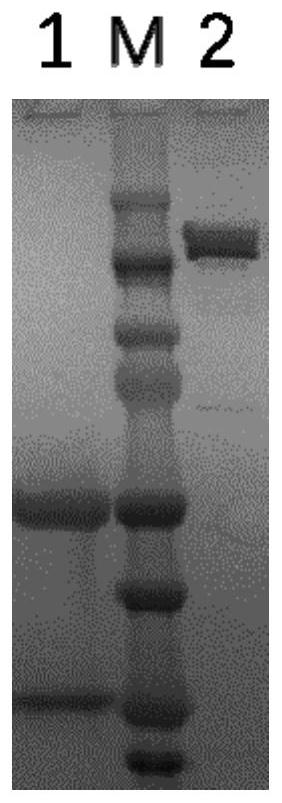
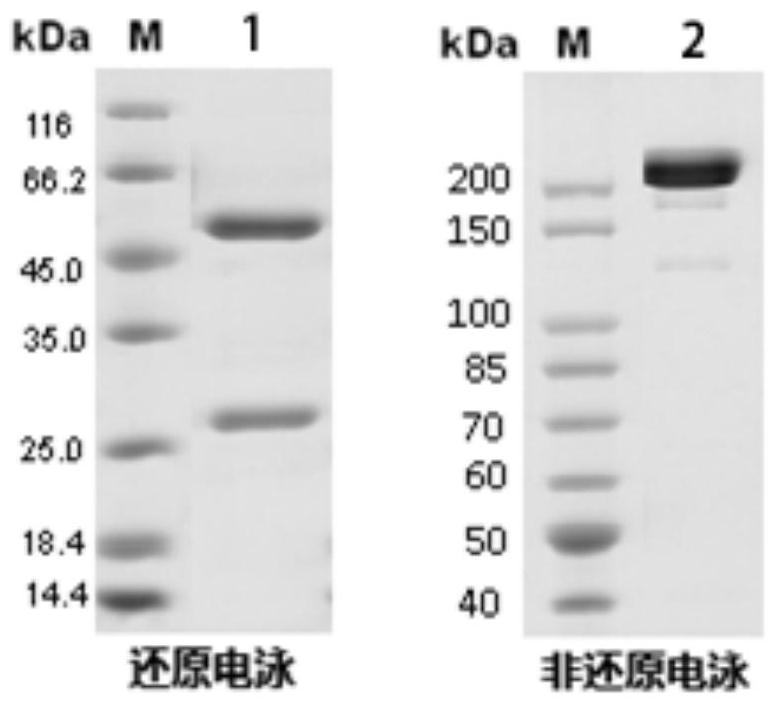
[0068] Example 3 Monoclonal Antibody and Miniculture and Purification
[0069] Take the six hybridoma cells listed in Table 8, transfer 1 mL of hybridoma cells into 100 mL culture flasks, and periodically add a certain amount of culture medium for cell expansion, and culture for 10 days. The supernatant was obtained by centrifugal filtration of the culture medium, and purified by Protein A affinity chromatography. The specific steps include:
[0070] After collecting the culture feed liquid and centrifuging at 6000rpm for 20min, filter it with a filter, and take the supernatant;
[0071] a. Pretreatment of chromatography column: The Protein A affinity chromatography column is rinsed with ultrapure water, and then equilibrated with equilibration buffer;
[0072] b. Sample loading and equilibration: The feed liquid is loaded onto the Protein A affinity chromatography column. After the sample is loaded, rinse with equilibration buffer until the baseline is stable;
[0073] c. E...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com