Florigen-activating complex
A florigen and compound technology, applied in compound screening, angiosperms/flowering plants, plant peptides, etc., can solve problems such as unclear mechanism of florigen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
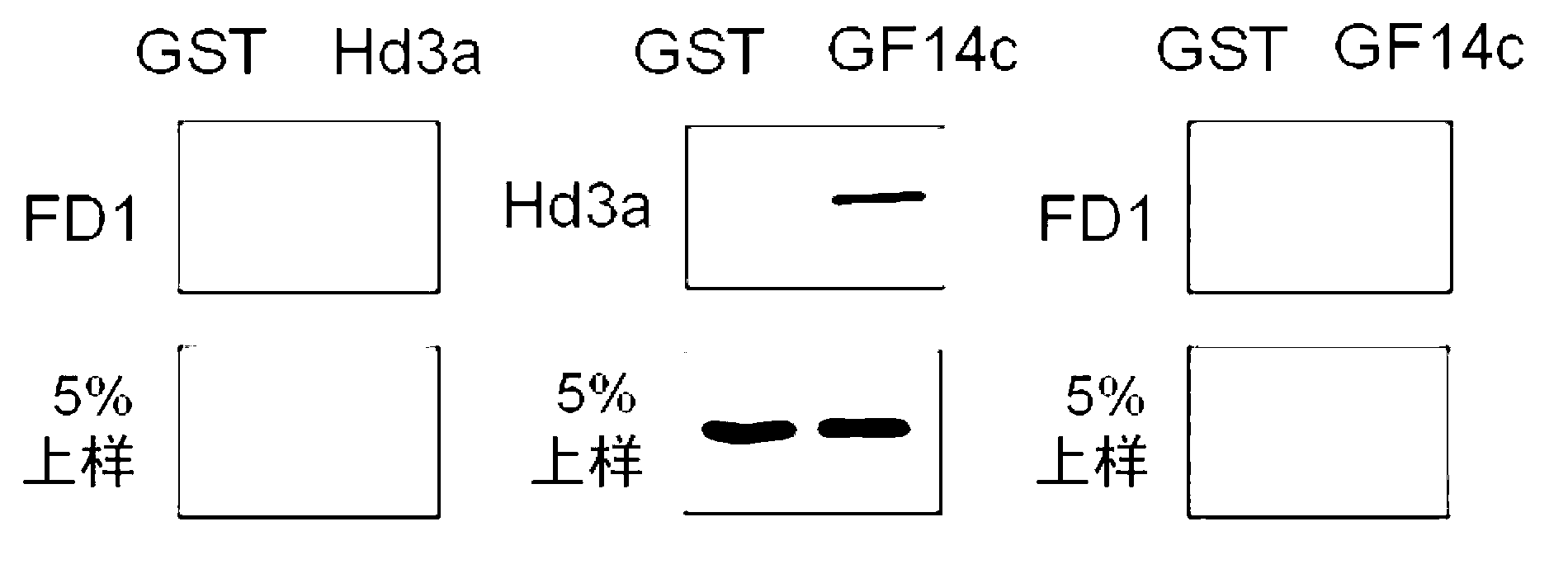
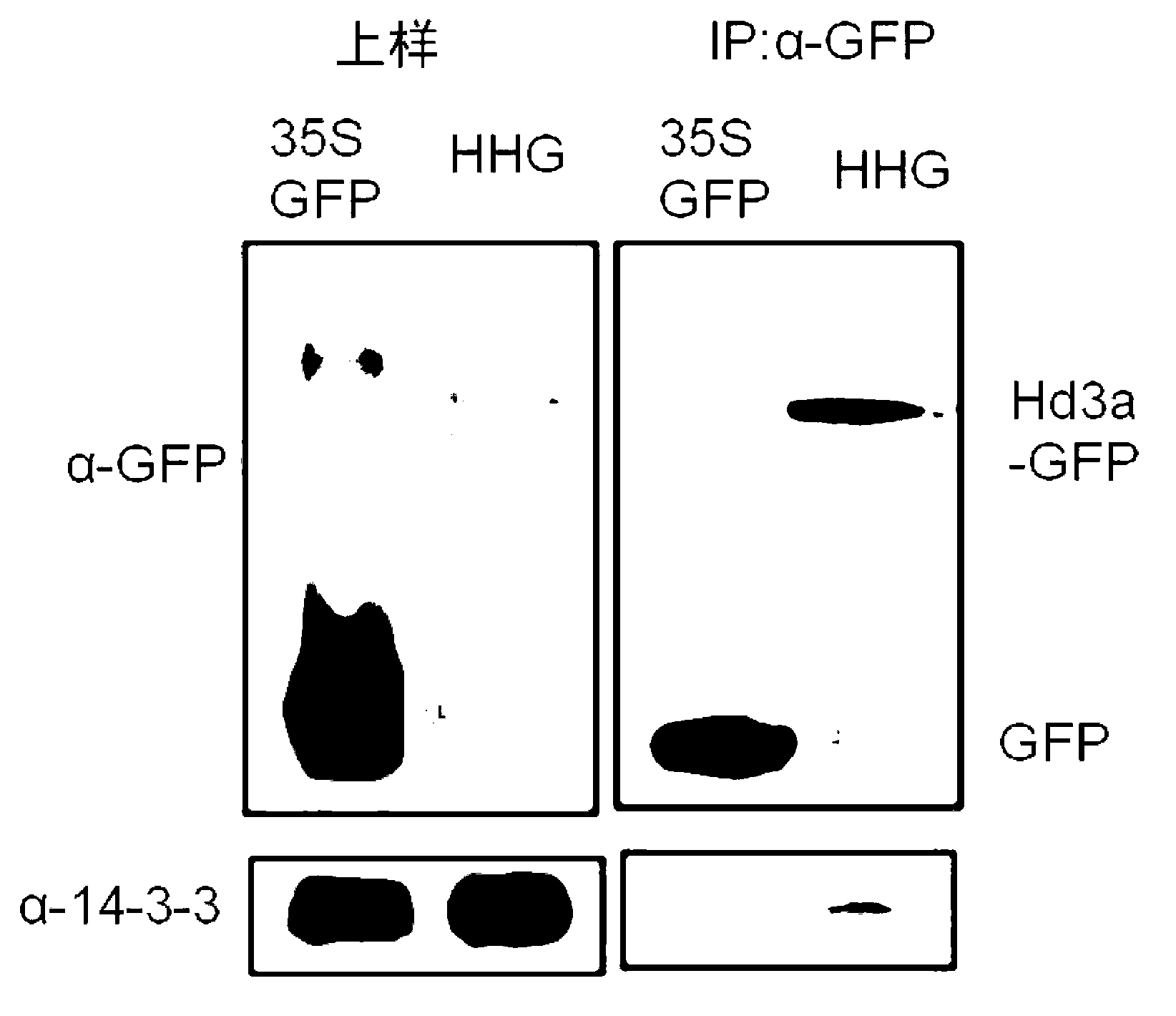
[0219] (Example 1) Confirmation of the interaction of three proteins
[0220] (1) The interaction of the three proteins was confirmed by in vitro GST pull-down experiments.
[0221] Restriction enzyme treatment was performed on the plasmid inserted with the cDNA encoding the full-length Hd3a (1-179 residues) shown in Sequence No. 1 and the cDNA encoding the full-length GF14c (1-256 residues) shown in Sequence No. 2, to make The fragment treated with the restriction enzyme was fused with the polynucleotide encoding GST and introduced into the vector. This vector was introduced into Escherichia coli, and GST fusion GF14c protein or GST fusion Hd3a protein was expressed in Escherichia coli. After the Escherichia coli is cultured, the Escherichia coli is centrifuged and recovered, and the GST fusion GF14c protein or the GST fusion Hd3a protein is separated and purified from the solution.
[0222] 10 nmol of the isolated and purified GST-fused GF14c protein or GST-fused Hd3a prot...
Embodiment 2
[0226] (Example 2) Preparation of crystals of florigen activation complex
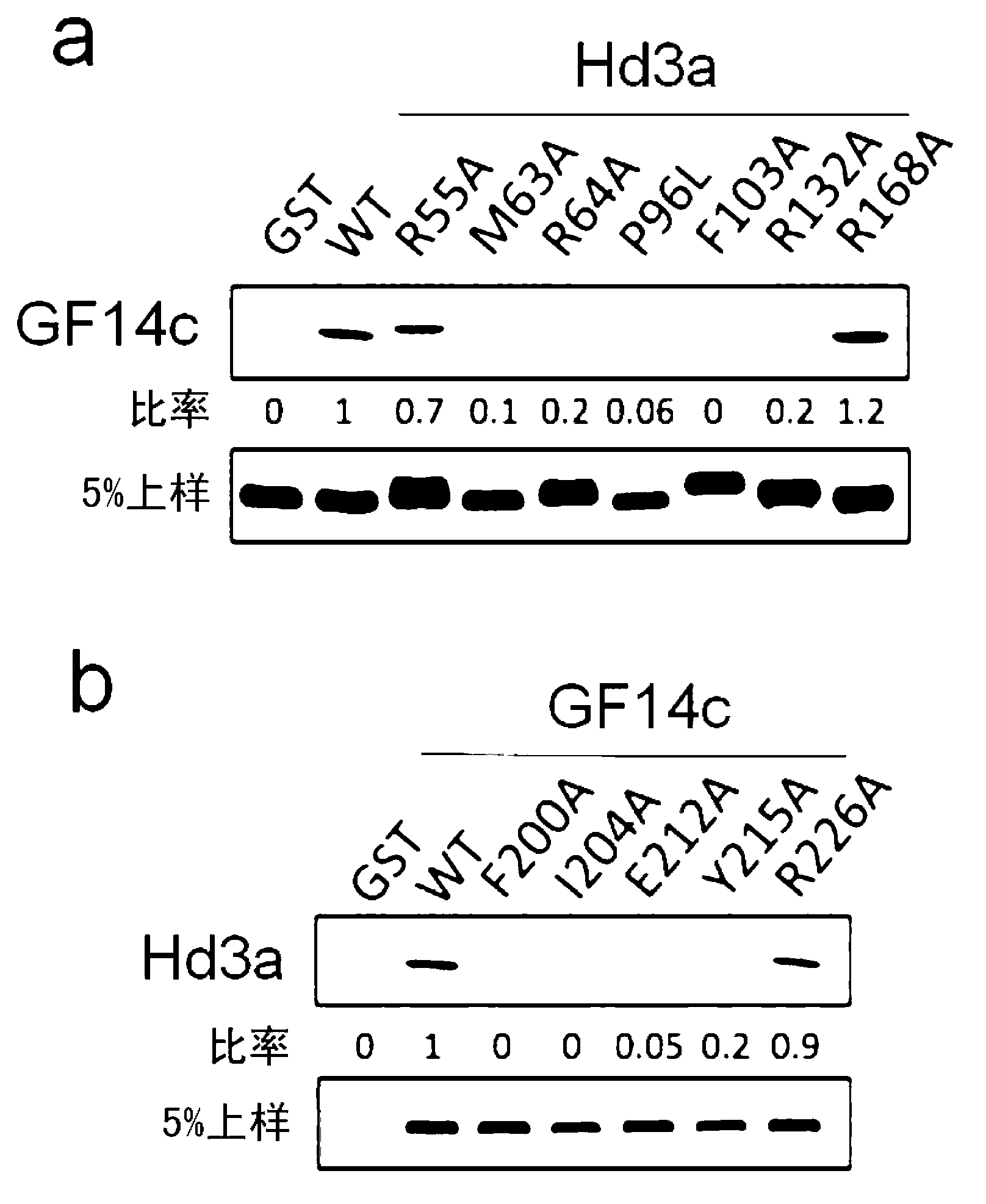
[0227] For the cDNA inserted with 6-170 residues in the full-length Hd3a (1-179 residues) shown in the coding sequence number 1, and 1-235 residues in the full-length GF14c (1-256 residues) shown in the coding sequence number 2 The cDNA plasmid of the residue was subjected to restriction enzyme treatment, and the fragment treated with the restriction enzyme was fused with a polynucleotide encoding GST to introduce it into a vector, and the vector was introduced into Escherichia coli. GST-fused Hd3a protein and GST-fused GF14c protein were expressed in Escherichia coli. After the E. coli was cultured, the E. coli was centrifuged and recovered, and the protein was purified from the lysed solution. Thereafter, the protein was analyzed by SDS-polyacrylamide electrophoresis (SDS-PAGE), and the purity of the protein was found to be 95% or more. A polypeptide encoding residues 187 to 195 of the full-length ...
Embodiment 3
[0235] (Example 3) Analysis of the X-ray structure of the crystal of the florigen-activated complex
[0236] The X-ray diffraction images of the obtained crystals were measured using a CCD detector at the BL5A beamline of Photon Factor, a radiological science research facility. The atomic coordinates of the florigen activation complex were obtained from the obtained diffraction images.
[0237] The X-ray diffraction data were collected, and the indexing of each diffraction spot and the calculation of the diffraction intensity were performed. The phase angle was determined from the obtained diffraction intensities and a search model using the molecular replacement method. Using the diffraction intensities and phase angles of these diffraction spots, an electron density map was derived by inverse Fourier transform. Atomic coordinates were constructed from the resulting electron density maps.
[0238] Specifically, the obtained data were processed using HKL2000 and scaled by S...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com