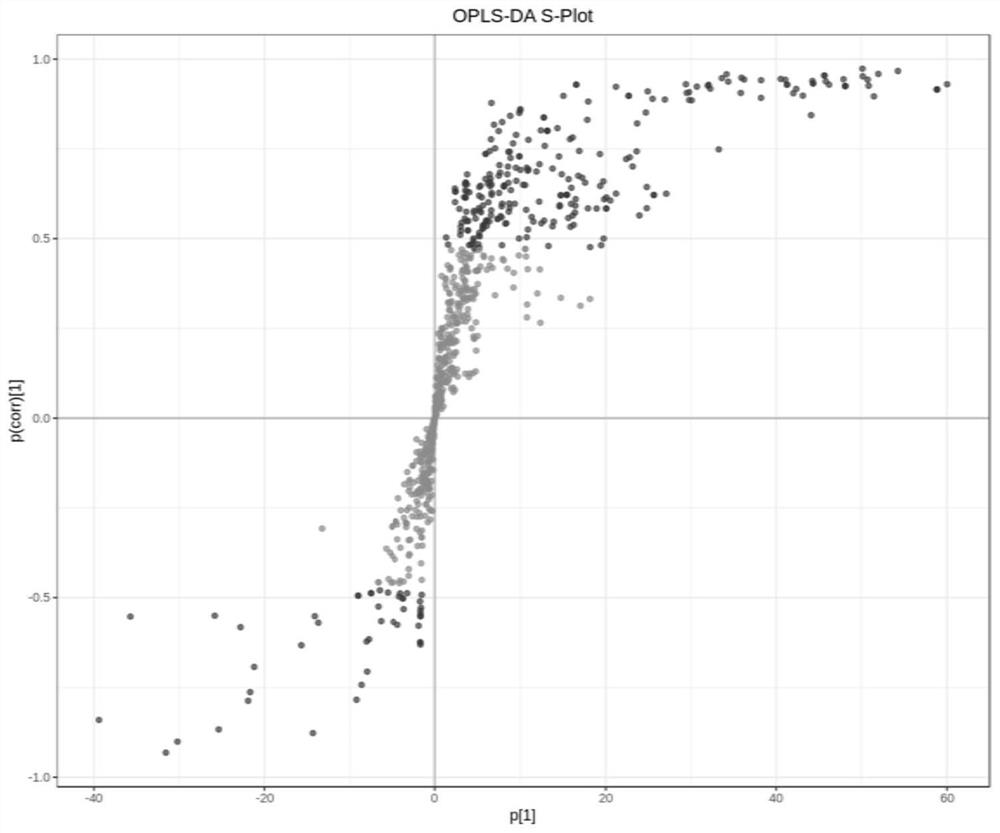
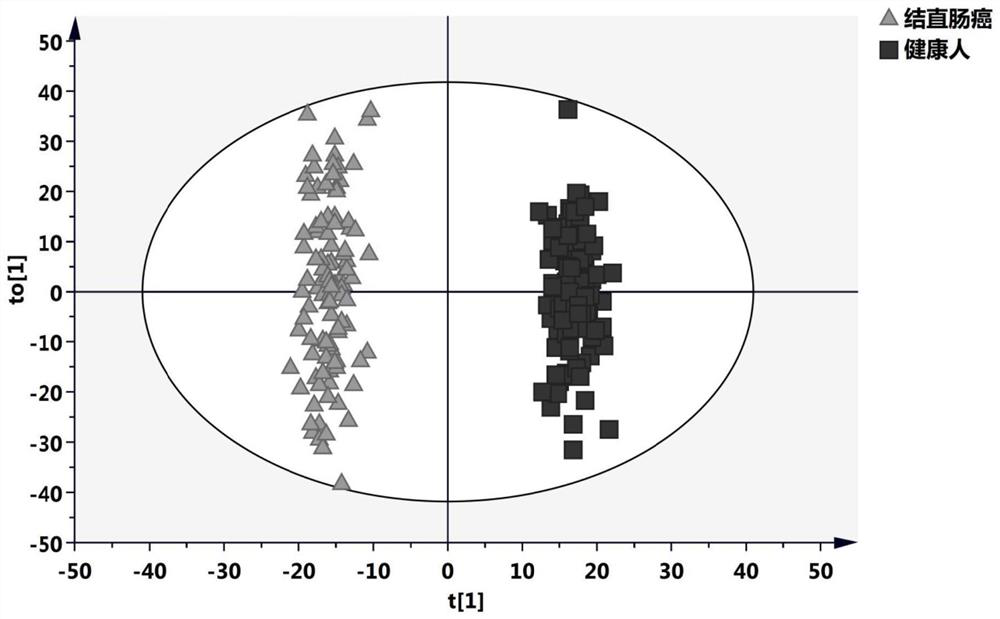
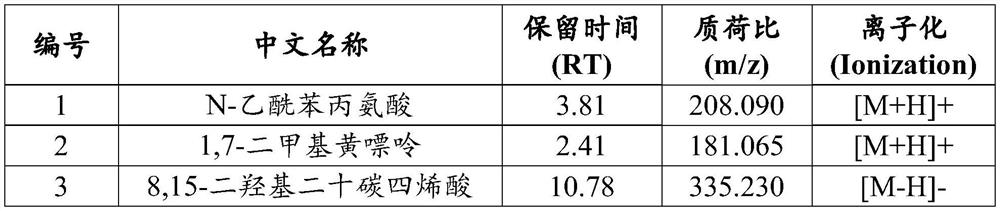
Plasma metabolism marker combination for diagnosing or monitoring colorectal cancer and application
A technology for metabolic markers and colorectal cancer, applied to the combination and application of plasma metabolic markers, can solve the problems of prone to false positives, poor sensitivity of plasma Septin9 gene methylation detection, and high missed diagnosis rate, so as to reduce trauma and missed diagnosis rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] This embodiment provides a method for screening plasma metabolic markers for diagnosing colorectal cancer, comprising the following steps:
[0029] S1, collect sample
[0030] In this study, peripheral venous blood plasma samples were collected from 100 healthy controls and 88 colorectal cancer patients from the Clinical Medical Research Center after obtaining patient consent. Among them, healthy controls were from people without intestinal diseases after physical examination; colorectal cancer patients were diagnosed by colorectal endoscopy and postoperatively. All samples had no history of any other malignant tumors, no other major systemic diseases, and no history of chronic diseases with long-term medication. The samples in each group were matched for age and sex.
[0031] Blood collection time was in the early morning on an empty stomach. All plasma samples were centrifuged and stored in a -80°C refrigerator. During the study, plasma samples were taken out and t...
Embodiment 2
[0069] Embodiment 2 Detection and verification
[0070] In this example, peripheral venous blood plasma samples from 405 healthy controls and 364 colorectal cancer patients from 2 independent medical research centers were collected after obtaining patient consent. Among them, healthy controls were from people without intestinal diseases after physical examination; colorectal cancer patients were diagnosed by colorectal endoscopy and postoperatively. All samples had no history of any other malignant tumors, no other major systemic diseases, and no history of chronic diseases with long-term medication. The samples in each group were matched for age and sex, and the blood collection time was in the early morning on an empty stomach. All plasma samples were centrifuged and stored in a -80°C refrigerator. During the study, plasma samples were taken out and thawed for subsequent analysis.
[0071] The detection conditions and data analysis methods in this example are the same as t...
Embodiment 3
[0087] This embodiment provides a detection kit for diagnosing or monitoring colorectal cancer, comprising:
[0088] (1) Standards for metabolic markers: N-acetylphenylalanine, 1,7-dimethylxanthine, 8,15-dihydroxyeicosatetraenoic acid, N-acetyl-5-methoxy Gelatin, 2-amino-4-hydroxypteridine, serotonin, lysophosphatidylcholine (15:0), methylmaleic acid, N-methyl-alpha-aminoisobutyric acid, thymine, alone Subpackage or mixed package.
[0089] (2) Solvent:
[0090] Pure methanol and 50% acetonitrile in water were used for sample extraction.
[0091] 50% acetonitrile in water can be used as a solvent for dissolving the standard.
[0092] (3) Internal standard: L-phenylalanine.
[0093] The screening method using the detection kit for diagnosing or monitoring colorectal cancer in this embodiment includes the following steps:
[0094] S1, collect plasma samples, preprocess, and obtain test solution.
[0095] S2, use LC-MS to analyze and detect the test solution to obtain N-acet...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com