Synthetic method of visible light mediated dihydroisoxazole
A technology of dihydroisoxazole and synthesis method, applied in the direction of organic chemistry, etc., can solve the problems of limited substrate range and severe reaction conditions, and achieve the effect of low price and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
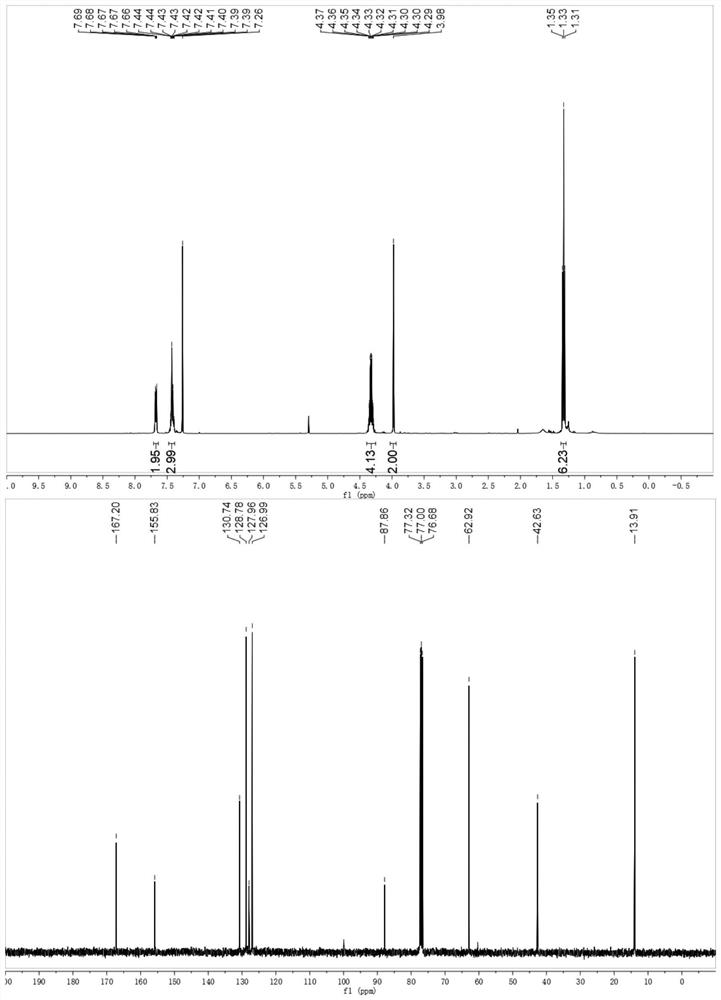
[0027] Example 1 Synthesis of 3-phenylisoxazole-5,5(4H)-diethyl carboxylate
[0028]
[0029] To a 10 mL Schlenk tube equipped with a stir bar was added styrene (0.2 mmol), diethyl dibromomalonate (0.48 mmol, 153 mg), fac-Ir (ppy) 3 (0.01 mmol, 6 mg), DMF (1 mL), DMAP (0.6 mmol, 73 mg) and tert-butyl nitrite (0.4 mmol, 41 mg). The Schlenk tube was evacuated and replaced 3 times with argon, then screwed on. The reaction mixture was subjected to visible light LED (λ max = 465 nm) under irradiation with stirring for 12 hours. The solvent was evaporated in vacuo and the residue was purified by flash column chromatography eluting with petroleum ether and ethyl acetate (5:1) to give the desired product in 79% yield.
[0030] H NMR spectrum of the product 3-phenylisoxazole-5,5(4H)-diethyl carboxylate: 1 H NMR (400MHz, CDCl 3 )δ7.80–7.57(m,2H),7.50–7.32(m,3H),4.44–4.18(m,4H),3.98(s,2H),1.33(,J=7.1Hz,6H).
[0031] Carbon spectrum: 13 C NMR (100MHz, CDCl 3 )δ167.2,155.8,130.7...
Embodiment 2
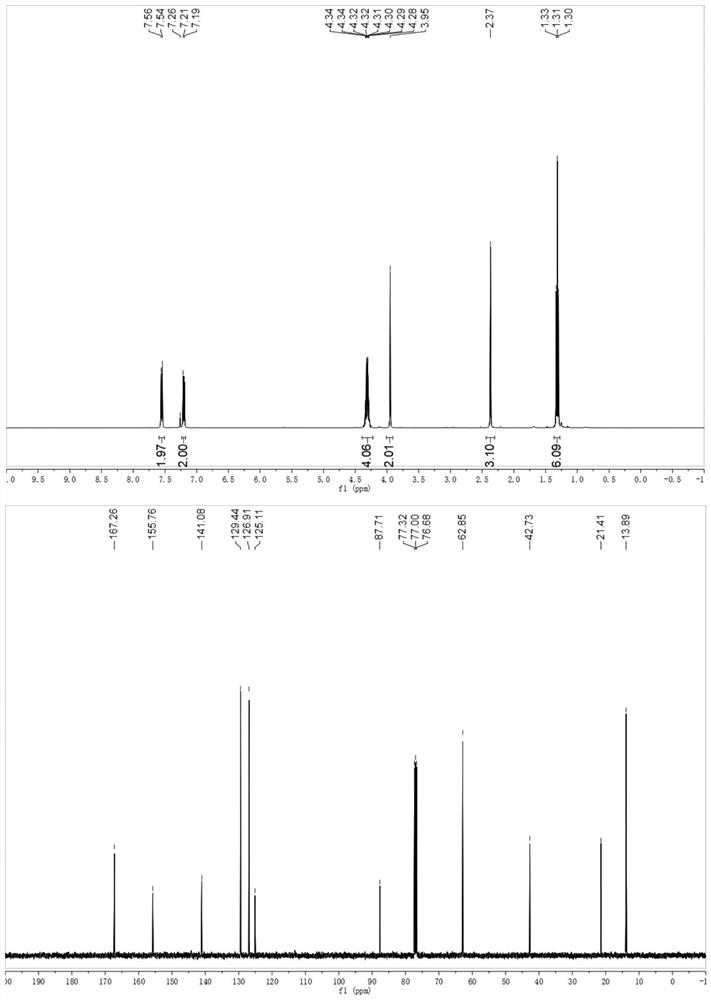
[0032] Example 2 Synthesis of 3-(p-tolyl)isoxazole-5,5(4H)-diethyl carboxylate
[0033]
[0034] To a 10 mL Schlenk tube equipped with a stir bar was added 4-methylstyrene (0.2 mmol), diethyl dibromomalonate (0.48 mmol, 153 mg), fac-Ir (ppy) 3 (0.01 mmol, 6 mg), DMF (1 mL), DMAP (0.6 mmol, 73 mg) and tert-butyl nitrite (0.4 mmol, 41 mg). The Schlenk tube was evacuated and replaced 3 times with argon, then screwed on. The reaction mixture was subjected to visible light LED (λ max = 465 nm) under irradiation with stirring for 12 hours. The solvent was evaporated in vacuo and the residue was purified by flash column chromatography eluting with petroleum ether and ethyl acetate (5:1) to give the desired product in 81% yield.
[0035] H NMR spectrum of the product 3-(p-tolyl)isoxazole-5,5(4H)-diethylcarboxylate: 1 H NMR (400MHz, CDCl 3 )δ7.55(d,J=8.2Hz,2H),7.20(d,J=8.0Hz,2H),4.39–4.22(m,4H),3.95(s,2H),2.37(s,3H), 1.31(,J=7.1Hz,6H).
[0036] Carbon spectrum: 13 C NMR (100...
Embodiment 3
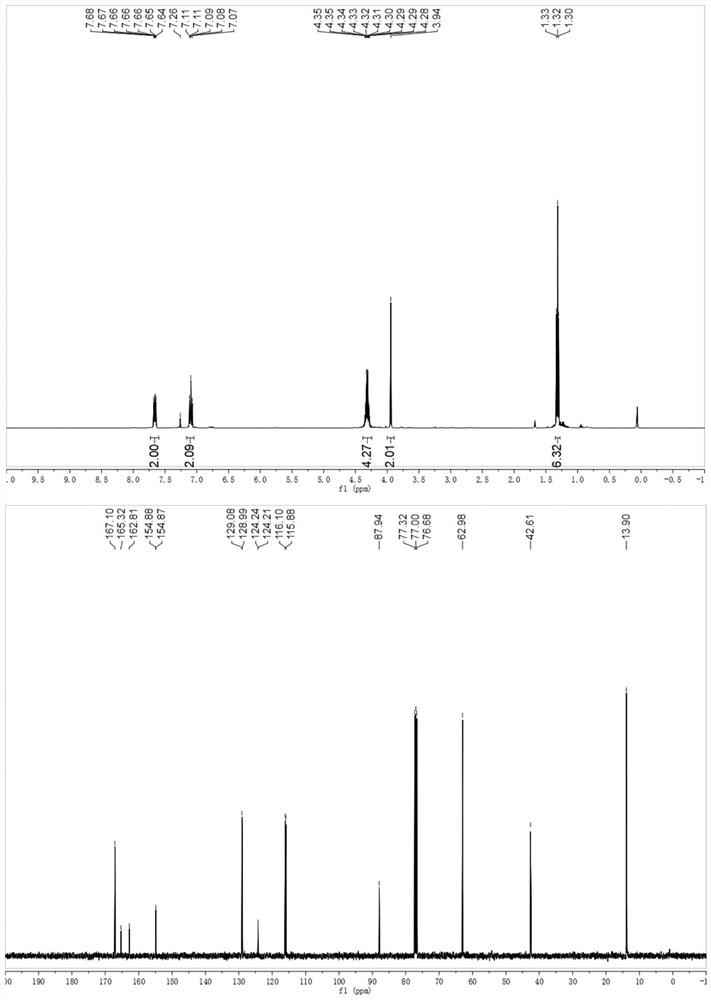
[0037] Example 3 Synthesis of 3-(4-methoxyphenyl)isoxazole-5,5(4H)-diethyl carboxylate
[0038]
[0039] To a 10 mL Schlenk tube equipped with a stir bar was added 4-methylstyrene (0.2 mmol), diethyl dibromomalonate (0.48 mmol, 153 mg), fac-Ir (ppy) 3(0.01 mmol, 6 mg), DMF (1 mL), DMAP (0.6 mmol, 73 mg) and tert-butyl nitrite (0.4 mmol, 41 mg). The Schlenk tube was evacuated and replaced 3 times with argon, then screwed on. The reaction mixture was subjected to visible light LED (λ max = 465 nm) under irradiation with stirring for 12 hours. The solvent was evaporated in vacuo and the residue was purified by flash column chromatography eluting with petroleum ether and ethyl acetate (5:1) to give the desired product in 81% yield.
[0040] H NMR spectrum of the product 3-(4-methoxyphenyl)isoxazole-5,5(4H)-diethylcarboxylate: 1 H NMR (400MHz, CDCl 3 )δ7.58(d,J=8.8Hz,2H),6.89(d,J=8.8Hz,2H),4.36–4.22(m,4H),3.92(s,2H),3.80(s,3H), 1.29(,J=7.1Hz,6H).
[0041] Carbon spectrum:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com