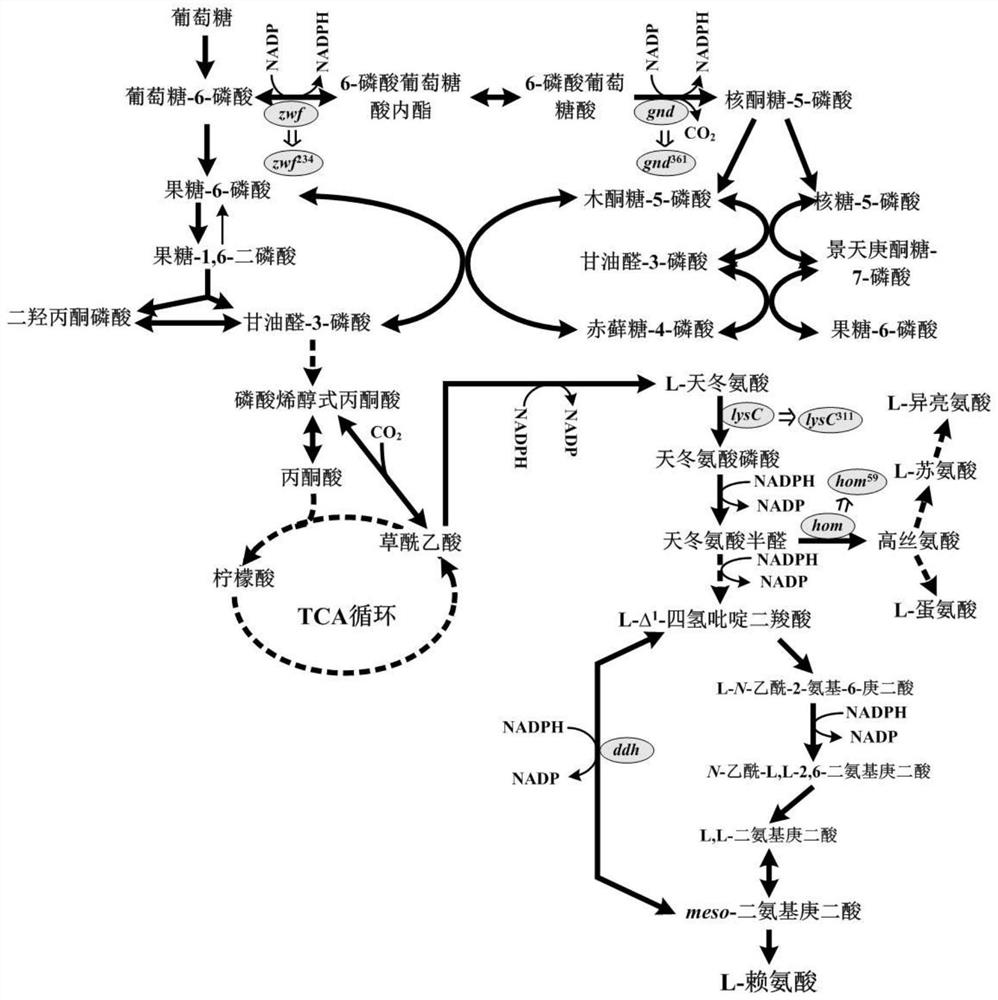
Construction and application of bacillus subtilis recombinant bacteria for producing L-lysine
A technology of Bacillus subtilis and recombinant bacteria, applied in the field of genetic engineering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: Plasmid pHT01-Cas9, pBE980b-hom 59 , pBE980b-lysC 311 , pBE980b-zwf 234 , pBE980b-gnd 361 and construction of pBE980b-ddh
[0039] Using the genome of Streptococcus pyogenes as a template, the gene Spcas9 was amplified by PCR using the primers Spcas9-F and Spcas9-R, and then the plasmid pHT01 and the fragment Spcas9 were double digested and ligated with restriction enzymes XbaI and XamI. , to obtain the target recombinant plasmid pHT01-Cas9.
[0040] pBE980b-hom 59 , pBE980b-lysC 311 , pBE980b-zwf 234 , pBE980b-gnd 361 and the construction of pBE980b-ddh using plasmid pBE980b as a template, using primers hom-F, hom-R, lysC-F, lysC-R, zwf-F, zwf-R, gnd-F, gnd-R, pksD-F and pksD-R seamlessly ligated 20bp sgRNA to plasmid pBE980b by inverse PCR amplification technology to obtain five plasmids with targeting sites.
[0041] Using B.subtilis ACCC11025 genome as a template, using primers hom-L-F, hom-L-R, hom-R-F, hom-R-R, lysC-L-F, lysC-L-R, lysC-R-F, lys...
Embodiment 2
[0042] Example 2: Screening and identification of target recombinant strains
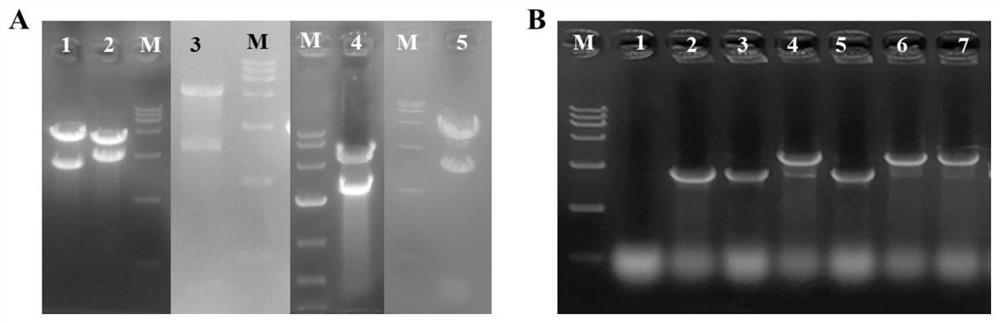
[0043] The recombinant plasmids pHT01-Cas9, pBE980b-hom 59 , pBE980b-lysC 311 , pBE980b-zwf 234 , pBE980b-gnd 361 Double-enzyme digestion or PCR verification was performed with pBE980b-ddh to determine the target recombinant plasmid. from figure 2 A shows that the selected plasmids all carry the target fragment and are the target recombinant plasmids. Subsequently, the plasmid pHT01-Cas9 was electroporated into B. subtilis ACCC11025, followed by the target recombinant plasmid pBE980b-hom 59 , pBE980b-lysC 311 , pBE980b-zwf 234 , pBE980b-gnd 361 and pBE980b-ddh were electroporated into B.subtilisACCC11025 competent cells with Cas9 protein. After PCR verification, the target heavy strain was confirmed ( figure 2 B).
Embodiment 3
[0044] Example 3: Growth, G6PD and 6GPD enzyme activities, and intracellular NADH concentration determination in recombinant bacteria and starting strains
[0045] Growth determination: take 200 μL of fermentation broth regularly, and dilute it to 5 mL with 0.25 mol / L dilute hydrochloric acid solution, then measure the absorbance at 562 nm with a UV spectrophotometer (ie: OD). 562 ).
[0046] Recombinant strain B. subtilis XH1 (ie: XH0 thrD::lysC 311 ) grew normally in LB solid medium supplemented with L-lysine structural analog S-(2-aminoethyl)-L-cysteine (AEC), while the starting strain B.subtilisXH0 could not grow ( image 3 B). These results suggest that replacing the thrD gene with lysC in B. subtilis 311The feedback regulation effect of L-lysine on AK III was successfully achieved.
[0047] It should be pointed out that compared with the starting strain B.subtilis XH0, the bacterial growth of the recombinant strain B.subtilis XH1 was not significantly inhibited, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com