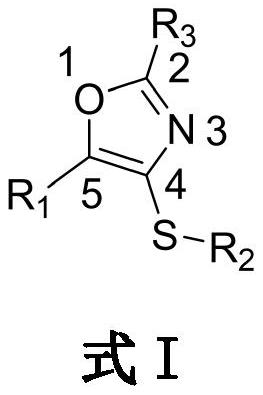
Sulfur-containing 2, 4, 5-trisubstituted oxazole compound as well as preparation method and application thereof
A technology for compounds and oxazoles, applied in the field of organic synthesis, can solve the problems of limited scope of substrate application and high price, and achieve the effects of overcoming the complex synthesis of precursors, high synthesis yield and wide applicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
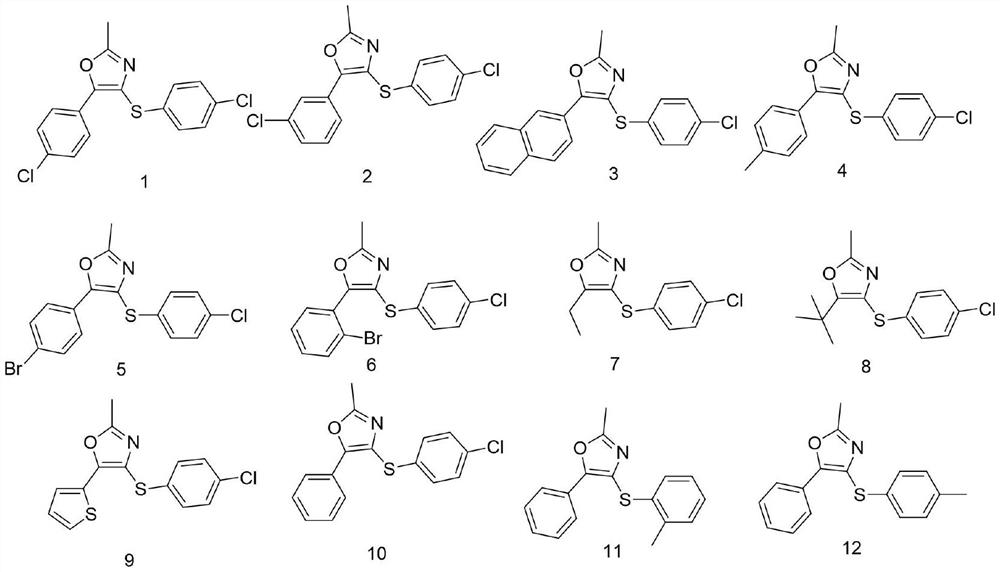
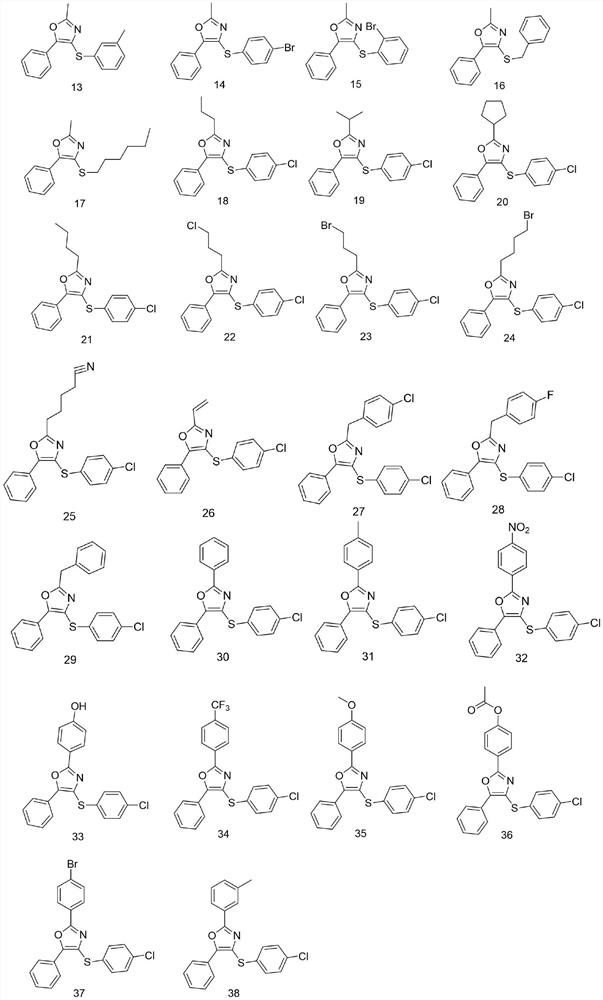
[0042] Example 1 Synthesis of Compound 1
[0043]
[0044] Step 1): add p-chlorostyrene (1.0 mmol), fluorine reagent Selectfluor (2.0 mmol) and 10 ml of methanol to a 100 ml round-bottomed flask, and stir at room temperature for 5 min. Then slowly add 10 ml of methanol solution containing 4-chlorothiophenol (1.5 mmol) dropwise, keep the temperature at 10 ~ 20 ° C and stir the reaction until the reaction is complete, after-treatment with ethyl acetate and saturated brine, The β-carbonyl sulfoxide 1' was isolated by column chromatography with a yield of 62%. Characterization of data: white solid (m.p.128-129℃); 1 HNMR(400MHz, Chloroform-d)δ 7.82(d,J=8.0Hz,2H),7.61(d,J=8.0Hz,2H),7.48(d,J=8.0Hz,2H),7.43(d,J =8.0Hz,2H),4.38(dd,J=14.4Hz,75.2Hz,2H); 13 C{ 1 H}NMR (100MHz, Chloroform-d) δ190.3, 141.6, 141.2, 138.2, 134.5, 130.4, 129.9, 129.4, 125.9, 65.8; IR (KBr): νmax 2945, 1675, 1385, 1204, 1047 (S=O), 821, 741, 495; HRMS (ESI, m / z) calcd.forC 14 H 10 Cl 2 NaO 2 S + [M...
Embodiment 2
[0047] Example 2 Synthesis of Compound 3
[0048]
[0049] Step 1): add 2-naphthalene vinylene (1.0 mmol), fluorine reagent Selectfluor (2.0 mmol) and 10 ml methanol to a 100 ml round-bottomed flask, and stir at room temperature for 5 min. Then slowly add 10 ml of methanol solution containing 4-chlorothiophenol (1.5 mmol) dropwise, keep the temperature at 10 ~ 20 ° C and stir the reaction until the reaction is complete, after-treatment with ethyl acetate and saturated brine, The corresponding β-carbonyl sulfoxide 3' was isolated by column chromatography with a yield of 81%. Characterization of data: white solid (m.p.116-117℃); 1 H NMR (400MHz, Chloroform-d) δ 8.36(s, 1H), 7.93(t, J=8.0Hz, 2H), 7.87(d, J=8.0Hz, 2H), 7.67-7.55(m, 4H), 7.47(d,J=8.0Hz,2H),4.57(dd,J=14.4Hz,104Hz,2H); 13 C{ 1 H}NMR (100MHz, Chloroform-d) δ 191.2, 141.9, 138.1, 136.2, 133.4, 132.4, 131.6, 130.0, 129.8, 129.5, 129.1, 128.1, 127.4, 126.0, 123.7, 66.2; IR (KBr): ν max 3057, 1651, 1469, 1292, 10...
Embodiment 3
[0052] Example 3 Synthesis of Compound 4
[0053]
[0054] Step 1): Add 4-methylstyrene (1.0 mmol), fluorine reagent Selectfluor (2.0 mmol) and 10 ml of methanol to a 100 ml round-bottomed flask, and stir at room temperature for 5 min. Then slowly add 10 ml of methanol solution containing 4-chlorothiophenol (1.5 mmol) dropwise, keep the temperature at 10 ~ 20 ° C and stir the reaction until the reaction is complete, after-treatment with ethyl acetate and saturated brine, The corresponding β-carbonyl sulfoxide 4' was isolated by column chromatography with a yield of 56%. Data characterization: white solid (m.p.89-90℃); 1 H NMR (400MHz, Chloroform-d) δ7.76(d, J=8.0Hz, 2H), 7.63(d, J=8.0Hz, 2H), 7.46(d, J=8.0Hz, 2H), 7.25(d , J=8.0Hz, 2H), 4.42 (dd, J=14.4 Hz, 102.8Hz, 2H); 13 C{ 1 H}NMR (100MHz, Chloroform-d) δ190.9, 145.7, 142.1, 137.9, 133.6, 129.8, 129.1, 126.0, 66.1, 22.0; IR(KBr):ν max 2948,1671,1385,1179,1047(S=O),808,498; HRMS(ESI,m / z)calcd.for C 15 H 13 ClNaO ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com