Eye drops and preparation method thereof
A technology of eye drops and chitosan derivatives, which is applied in the direction of pharmaceutical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problem of affecting the balance of eye flora and aggravating eye health Effects, eye drop dependence and other problems, to achieve the effect of assisting in maintaining ocular surface health, promoting wound healing, and relieving pain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
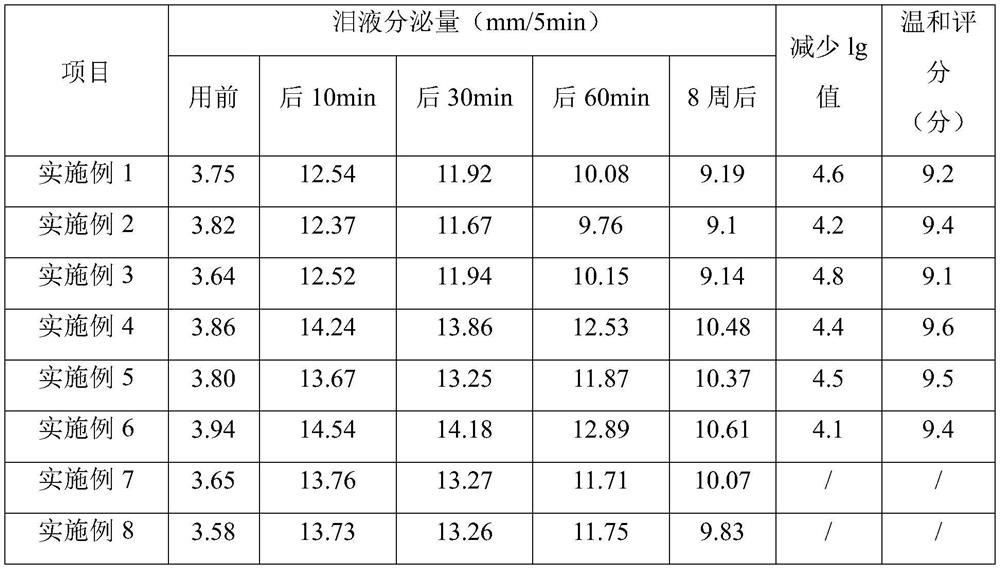
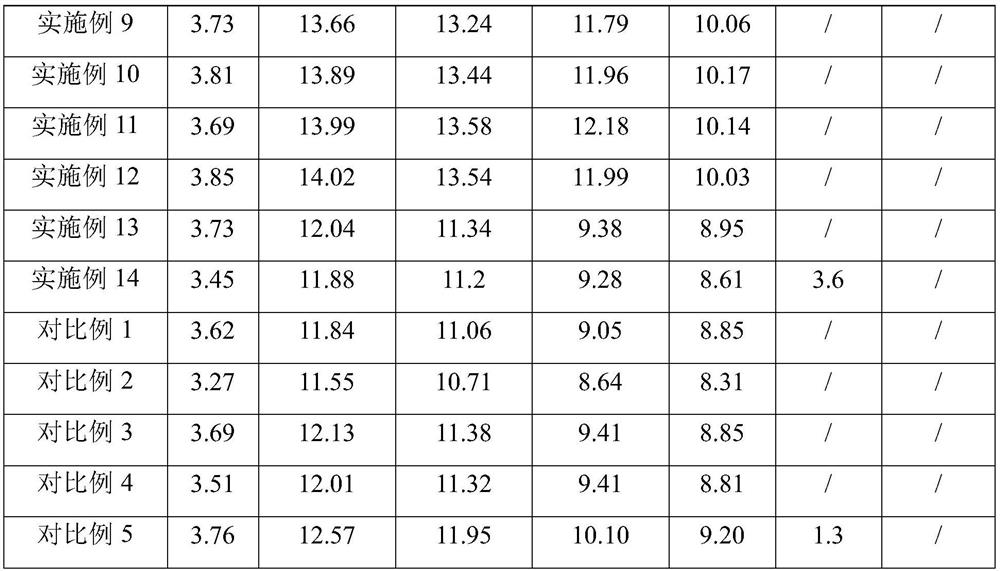
Image
Examples
Embodiment 1
[0041] Example 1: An eye drop:
[0042] Chitosan derivative 0.25kg, hydroxypropyl methylcellulose 0.25kg, sodium hyaluronate 0.3kg, osmotic pressure regulator 0.9kg, chelating agent 0.25kg, bacteriostatic agent 0.008kg, sodium perborate tetrahydrate 0.005 kg, propylene glycol 0.5kg, boric acid 3kg, water 95kg; chitosan derivative is carboxymethyl chitosan; osmotic pressure regulator is composed of NaCl and KCl with a weight ratio of 1:2; bacteriostatic agent is composed of a weight ratio of 1 : 1 phenoxyethanol and ethyl hydroxyphenyl are formed; the chelating agent is EDTA-2Na;
[0043] The preparation method is as follows:
[0044] S1, weigh sodium perborate tetrahydrate, boric acid and mix and stir to obtain a premix;
[0045] S2. Weigh water and heat it to 85°C, then add hydroxypropyl methylcellulose and stir to dissolve, and then let stand at 35°C to obtain a primary mixture;
[0046] S3. Weigh the chitosan derivative and add it to the initial mixture and stir it evenl...
Embodiment 2
[0048] Embodiment 2: The difference between this embodiment and Embodiment 1 is:
[0049] Chitosan derivative 0.01kg, hydroxypropyl methylcellulose 0.01kg, sodium hyaluronate 0.01kg, osmotic pressure regulator 0.5kg, chelating agent 0.01kg, bacteriostatic agent 0.005kg, sodium perborate tetrahydrate 0.002 kg, propylene glycol 0.1kg, boric acid 0.1kg, water 85kg; the chitosan derivative is hydroxyethyl chitosan; the osmotic pressure regulator is composed of NaCl and KCl with a weight ratio of 1:1; the bacteriostatic agent is composed of a weight ratio of 1:0.6 composition of phenoxyethanol and ethyl hydroxyphenyl; chelating agent is EDTA-2Na;
[0050] During preparation:
[0051] S2. Weigh the water and heat it to 80°C, then add hydroxypropyl methylcellulose and stir to dissolve, and then let stand to 30°C to obtain the initial mixture;
Embodiment 3
[0052] Embodiment 3: The difference between this embodiment and Embodiment 1 is:
[0053] Chitosan derivative 0.5kg, hydroxypropyl methylcellulose 0.5kg, sodium hyaluronate 0.5kg, osmotic pressure regulator 1.5kg, chelating agent 0.5kg, bacteriostatic agent 0.01kg, sodium perborate tetrahydrate 0.01 kg, propylene glycol 2kg, boric acid 5kg, water 100kg; the chitosan derivative is succinyl chitosan; the osmotic pressure regulator is composed of NaCl and KCl with a weight ratio of 1:3; the bacteriostatic agent is composed of a weight ratio of 1:1.5 The composition of phenoxyethanol and ethyl paraben; the chelating agent is EDTA-2Na.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com