Preparation method of sheep peripheral blood lymphocyte transfer factor solution and injection
A technology of lymphocytes and transfer factors, which is applied in the preparation methods of peptides, cytokines/lymphokines/interferons, blood/immune system cells, etc., to achieve the effect of increasing production and promoting a wide range of applications.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] A preparation method of a sheep peripheral blood lymphocyte transfer factor solution, comprising the following steps:
[0026] Step 1: Preparation of Lymphocyte Seed Suspension:
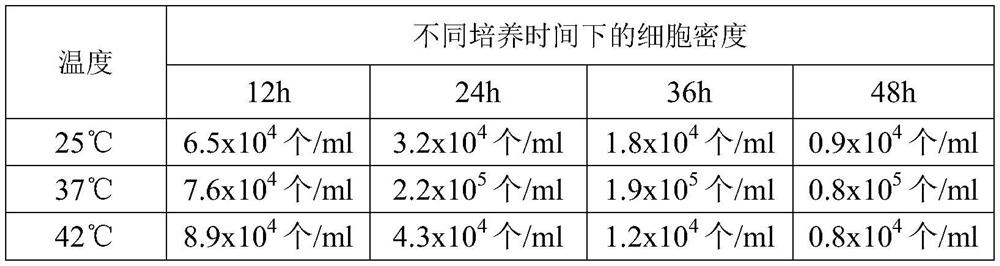
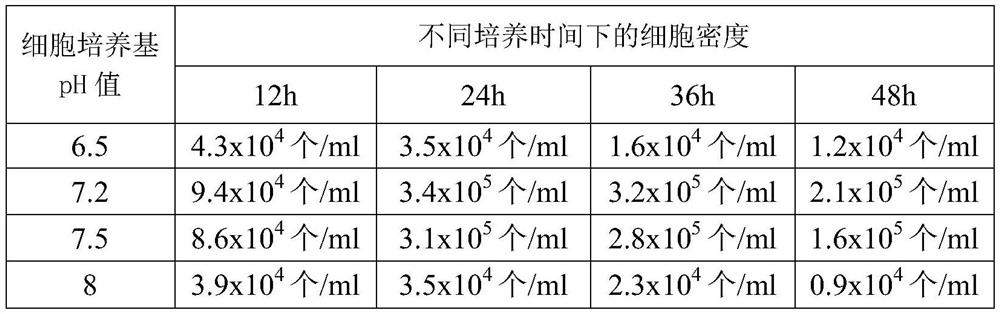
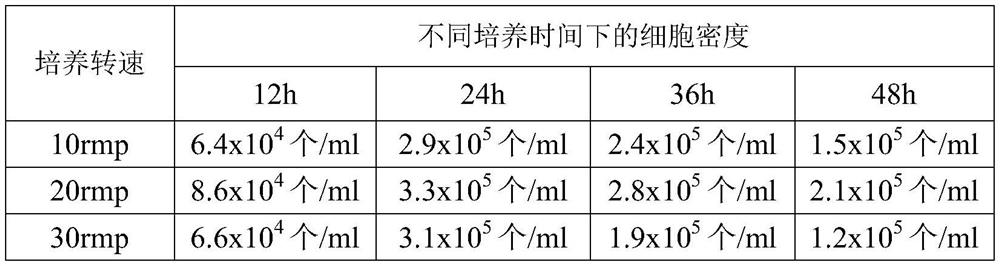
[0027] (1) Collection of fresh sheep blood: Donor sheep should meet the current "Veterinary Pharmacopoeia of the People's Republic of China" on animal standards for production and inspection. After the sheep is sterilized, insert the blood collection needle into the sheep jugular vein, and aseptically collect blood into the blood collection bottle. Anticoagulation is available.
[0028] (2) Cell sedimentation and separation: Add 1 volume of anticoagulant physiological saline to the above anticoagulant and mix well, then dispense into each separatory funnel (1L), and settle naturally on the ultra-clean workbench for 24 hours . After 24 hours, flip the switch of the separatory funnel to remove the red blood cells at the bottom of the separatory funnel.
[0029] (3) Preparation of lymphocyte ...
Embodiment 2
[0051] For a transfer factor injection, the transfer factor solution obtained in Example 1 (obtained after culturing with optimal culture conditions) is sterile filtered using a 0.22 μm filter element. Then add mannitol and normal saline, mannitol: normal saline: transfer factor solution according to the volume ratio of 2:2:6 to prepare a transfer factor solution with a polypeptide content of not less than 1.5mg / ml and a ribose content of not less than 45ug / ml for injection liquid.
Embodiment 3
[0053] 1. Quality standard detection of transfer factor injection
[0054] According to the current appendix of "Veterinary Pharmacopoeia of the People's Republic of China", it should be aseptically grown. According to the determination method of polypeptide content in the quality standard of transfer factor oral liquid in the 2017 edition of "Veterinary Drug Quality Standard Biological Products Volume", we prepared three batches of transfer factor injection, and three batches of reference substances (purchased from Qingdao Yibang Bioengineering Co., Ltd. The company's pig spleen transfer factor injection) has been tested for polypeptide content. The content of the transfer factor polypeptide prepared by the present invention is not less than 1.5 mg per milliliter, which meets the requirements of veterinary drug quality standards, and the polypeptide content is higher than that of the reference substance (see Table 4 for the results). ).
[0055] Table 4 Detection and compari...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com