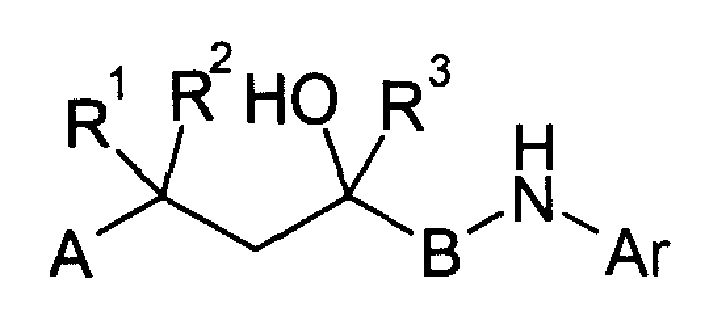
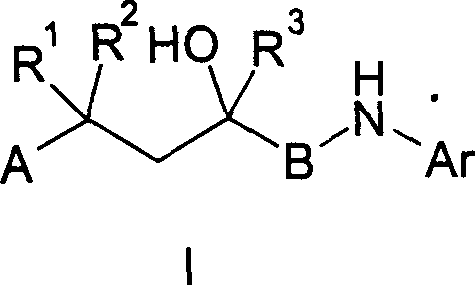
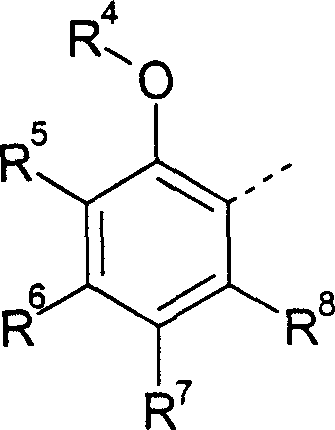
Nonsteroidal antiinflammatories
An inflammation and drug technology, applied in the field of non-steroidal compounds, which can solve the problems of impossible anti-inflammatory effect and metabolic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0329] Embodiment 1 (method 1)
[0330] 5-{2-Hydroxy-3-[1-(2-methoxyphenyl)-cyclopropyl]-2-trifluoromethyl-propionamido}-o-hydroxymethylbenzoic acid lactone
[0331] 500 mg of 5-{3-[1-(2-methoxyphenyl)-cyclopropyl]-2-oxo-propionamido}-o-hydroxymethylbenzoic acid lactone was dissolved in 15 mL of di methylformamide, and mixed with 0.77 ml of trifluoromethyl-trimethylsilane and 500 mg of cesium carbonate under ice cooling. After stirring at room temperature for 18 hours, 5 ml of a 1 mol / L tetrabutylammonium fluoride solution in tetrahydrofuran was added, a few drops of water were added, and the mixture was stirred at room temperature for one hour. After addition of 100 ml of water, extraction is carried out with ethyl acetate, the organic phase is dried over sodium sulfate and concentrated by evaporation. The crude product is chromatographed on silica gel. Elution with hexane / ethyl acetate (80:20) afforded the pure title compound.
[0332] The mixture o...
Embodiment 2
[0336] 5-{3-[1-(5-Fluoro-2-methoxyphenyl)-cyclopropyl]-2-hydroxy-2-trifluoromethyl-propionamido}-o-hydroxymethylbenzoic acid lactone
[0337] By 5-{3-[1-(5-fluoro-2-methoxyphenyl)-cyclopropyl]-2-oxo-propionamido}-o-hydroxymethylbenzoic acid lactone by similar to Example 1 The method is obtained, its flash point is 170-179 ° C (racemate).
Embodiment 3
[0338] Embodiment 3 (method 2)
[0339] 5-[2-Hydroxy-4-(2-methoxyphenyl)-4-methyl-2-trifluoromethyl-pentanamido]-o-hydroxymethylbenzoic acid lactone
[0340] 400 mg of 2-hydroxy-4-(2-methoxyphenyl)-4-methyl-2-trifluoromethyl-pentanoic acid in 5 mL of dimethylacetamide at 0 °C with 0.23 mL of thionyl chloride mix. After stirring at 0°C for 30 minutes, 390 mg of 5-aminophthalide in 2 ml of dimethylacetamide were added and the mixture was stirred at this temperature for a further 4 hours. Then ice water was added, extraction was carried out with ethyl acetate, and after drying over sodium sulfate, the crude product was chromatographed on silica gel. Elution with hexane / ethyl acetate (50:50) gave the title compound as crystals with a flash point of 134-135°C.
[0341] After separation of the racemate, the obtained
[0342] (+)-enantiomer with a flash point of 135-136°C, [α] D +192.2° (c=1, CHCl 3 )
[0343] (-)-enantiomer with a flash point of 136-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Flash point | aaaaa | aaaaa |
| Flash point | aaaaa | aaaaa |
| Flash point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com