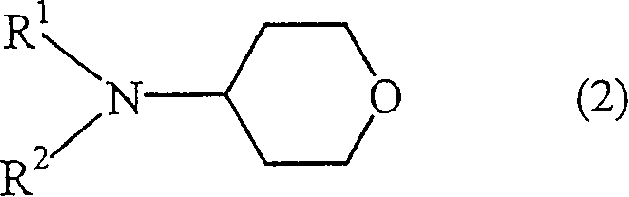
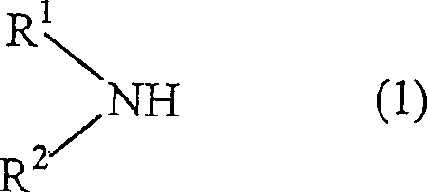
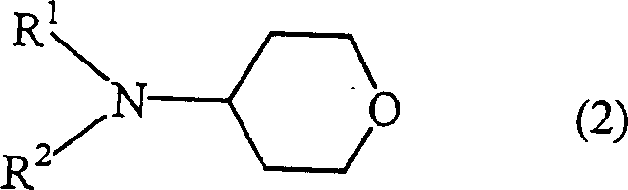
Processes for producing tetrahydropyranyl-4 sulfonate and 4-amino tetrapyran compound
A technology of aminotetrahydropyran compound and tetrahydropyranyl, which is applied in the field of producing 4-aminotetrahydropyran compound and producing tetrahydropyranyl-4-sulfonate, and can solve the problem of tetrahydropyran compound Problems such as low yield, low yield, and difficult synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] 1.00 g (13.9 mmol) of 3-buten-1-ol and 1.35 g (16.6 mmol) of 37% by weight aqueous formaldehyde (from Wako Junyaku Co. obtained), under a nitrogen atmosphere, 2.66 g (27.7 mmol) of methanesulfonic acid was added dropwise with stirring, and the resulting mixture was reacted at 25° C. for 3 hours. When the reaction was completed, the resulting reaction mixture was analyzed by high-performance liquid chromatography, and it was found that 1.65 g (yield: 66%) of tetrahydropyranyl-4-methanesulfonate had been formed.
Embodiment 2
[0050] In a reaction apparatus similar to Example 1, 1.00 g (13.9 mmol) of 3-buten-1-ol, 0.50 g (15.3 mmol) of 92% by weight paraformaldehyde (obtained from Mitsui Toatsu Chemical Co.) and 5ml of toluene, under a nitrogen atmosphere, 3.99g (41.5mmol) of methanesulfonic acid was added dropwise with stirring, and the resulting mixture was reacted at 25°C for 3 hours. When the reaction was completed, the resulting reaction mixture was analyzed by high-performance liquid chromatography to find that 2.15 g (yield: 86%) of tetrahydropyranyl-4-methanesulfonate had been formed.
Embodiment 3
[0052] Add 1.00g (13.9mmol) 3-buten-1-ol, 0.50g (5.6mmol, calculated as formaldehyde, it is equivalent to 16.8mmol) trioxane and 5ml Toluene, under a nitrogen atmosphere, 2.66 g (27.7 mmol) of methanesulfonic acid was added dropwise with stirring, and the resulting mixture was reacted at 25° C. for 3 hours. When the reaction was completed, the resulting reaction mixture was analyzed by high performance liquid chromatography to find that 2.13 g (yield: 85%) of tetrahydropyranyl-4-methanesulfonate had been produced.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com