Tumor-associated antigen derivatives from MAGE family, and nucleic acid sequence encoding them
A tumor-related, nucleic acid sequence technology, used in tumor-specific antigens, tumor rejection antigen precursors, anti-tumor drugs, etc., can solve problems such as inability to detect antigens
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment I
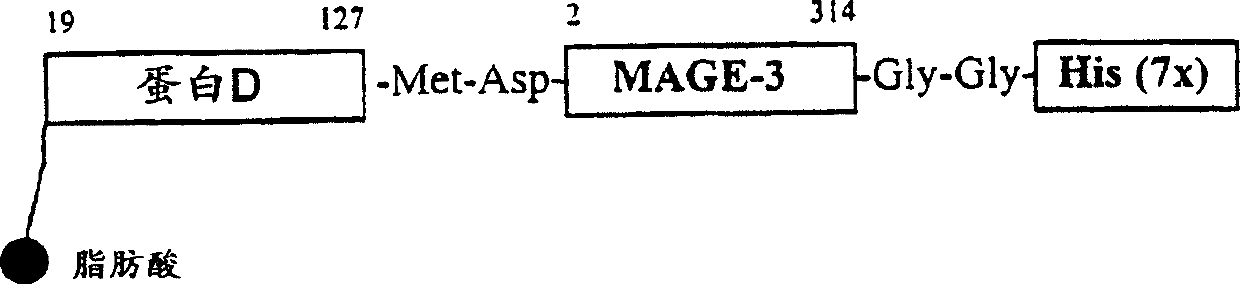
[0087] A recombinant Escherichia coli strain expressing the fusion protein lipoprotein D-MAGE-3-His (LPD 1 / 3-MAGE-3-His or LpD MAGE-3-His) was prepared.
[0088] 1. Escherichia coli expression system:
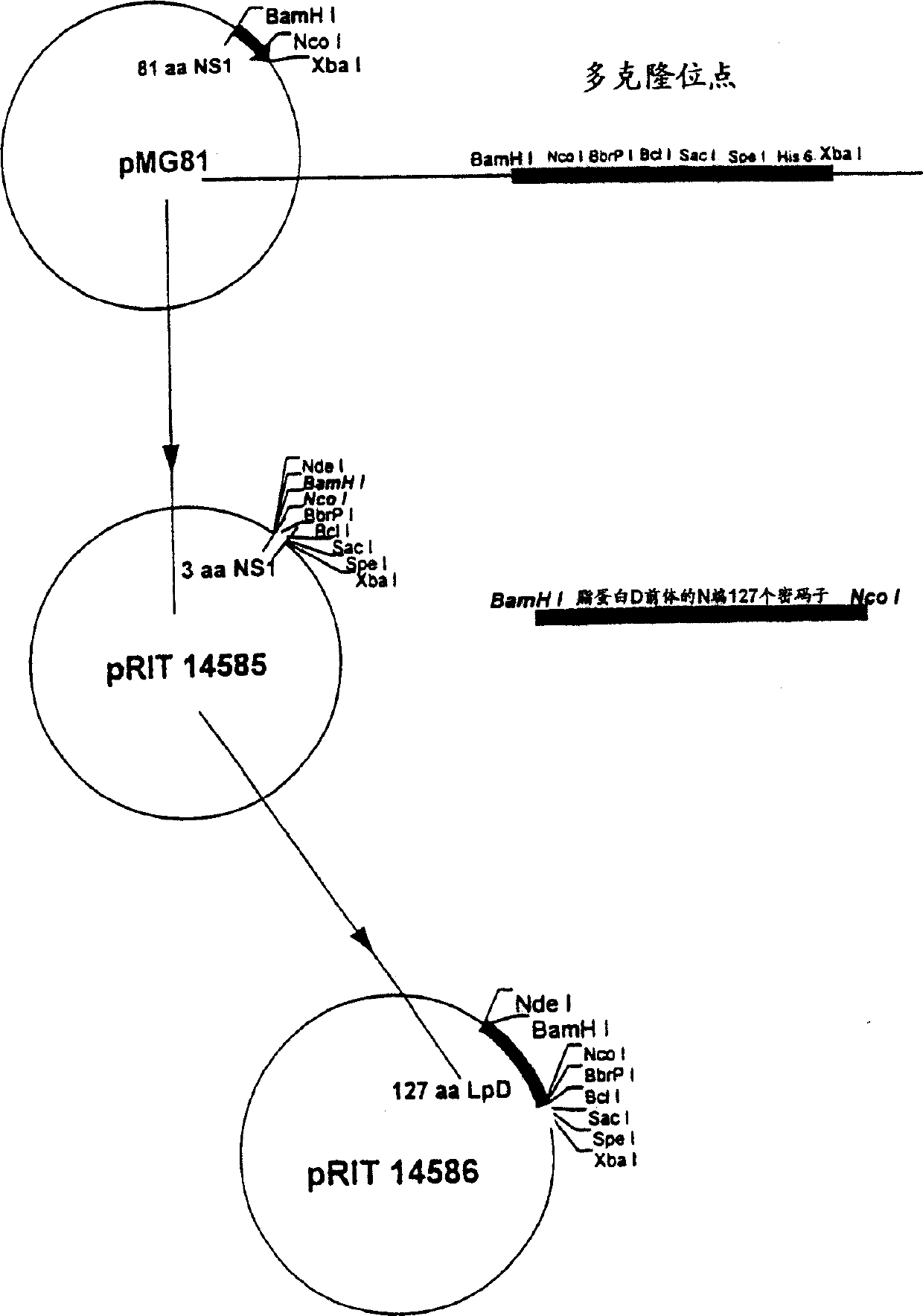
[0089] To produce lipoprotein D, DNA encoding protein D has been cloned into the expression vector pMG81. This plasmid utilizes signals from λ-phage DNA to drive the transcription and translation of the inserted foreign gene. This vector contains the λPL promoter PL, the operator OL and two utilization sites (NutL and NutR) to depolarize transcription when N protein is provided (Gross et al., 1985, Molecular Cell Biology, 5:1015) . A vector containing the PL promoter was introduced into an E. coli lysogenic host in order to stabilize the plasmid DNA. Lysogenic host strains contain replication-defective lambda phage DNA integrated into the genome (Shatzman et al., 1983, in "Experimental Manipulation of Gene Expression", Inouya (Ed.), PP 1-14, Academic Press, NY). The λ-phage...
Embodiment II
[0107] Preparation of LPD 1 / 3-MAGE-3-His antigen:
[0108] 1. Cultivation and induction of strains - expression of LPD 1 / 3-MAGE-3-His:
[0109] AR58 cells transformed with plasmid pRIT 14477 were cultured in 2 liter shake flasks, each containing 400ml of LY 12 medium supplemented with yeast extract (6.4g / L) and kanamycin sulfate (50mg / L) . After incubation for 8±1 hours at 30°C on a shaker, a small sample was removed from each shaker flask for microscopic examination. The contents of the two shake flasks were combined to provide the inoculum to a 20 liter fermenter.
[0110] This inoculum (approximately 800 ml) was added to a pre-sterilized 20 liter (total volume) fermenter containing 7 liters of medium supplemented with 50 mg / L kanamycin sulfate. By timing the addition of NH 4 OH (25% v / v) calibrated and maintained pH at 6.8, and calibrated and maintained temperature at 30°C. The aeration rate was calibrated and maintained at 12 L / min using agitation rate feedback contro...
Embodiment III
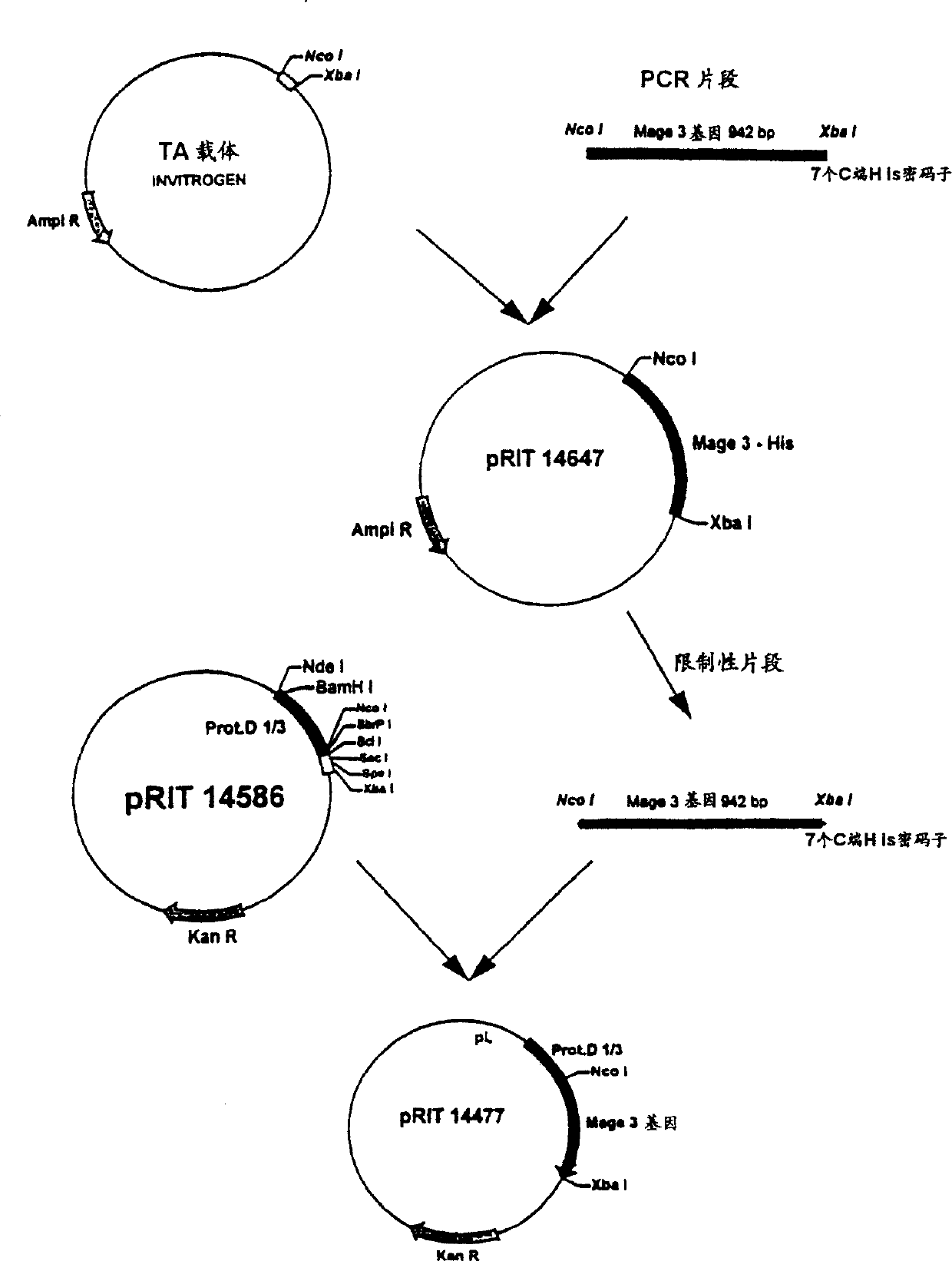
[0119] Identification of fusion protein Lipo D-MAGE 3:
[0120] 1. Purification:
[0121] LPD-MAGE-3-His was purified from cell homogenate using the following sequence of steps:
[0122] a) - solubilization of the washed pellet fractions from the cell lysate,
[0123] b) - Chemical reduction of intramolecular and intermolecular disulfide bonds in proteins, with subsequent blocking of sulfhydryl groups to prevent oxidative recoupling,
[0124] c) - microfiltration of the reaction mixture in order to remove particulates and reduce endotoxins,
[0125] d) - Capture and preliminary purification of LPD-MAGE-3-His using the affinity interaction between polyhistidine tails and zinc-loaded chelating agarose,
[0126] e) - Removal of contaminating proteins by anion exchange chromatography.
[0127] This purified LPD-MAGE-3-His was subjected to several polishing steps:
[0128] f) - buffer exchange / urea scavenging by size exclusion chromatography using Superdex 75,
[0129] g) - f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com