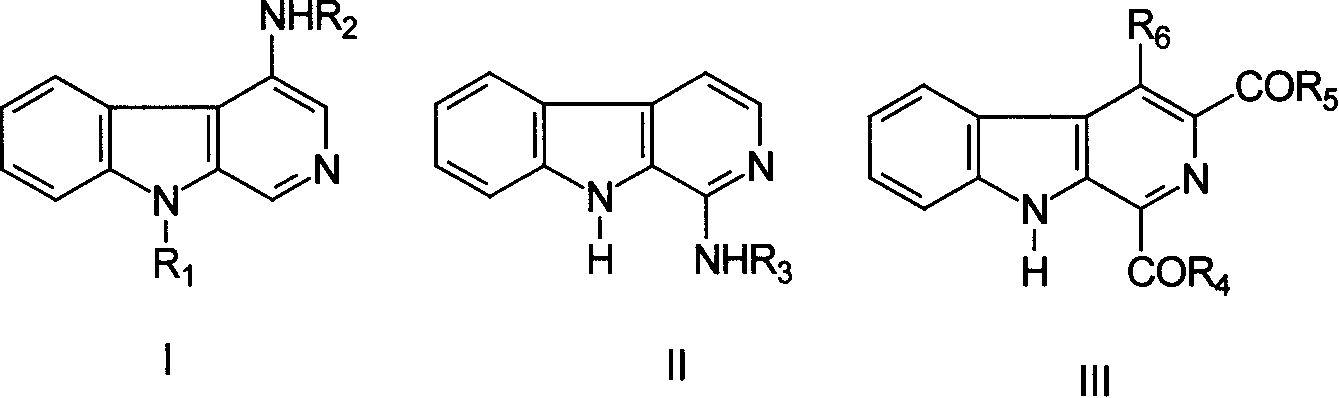
Beta-carboline and thio-analogues subsitituted by amino-acid radical/acyl, preparing method and application thereof
An acyl substitution and amino technology, applied in the field of drug synthesis, can solve the problem of the lack of ability to stabilize the DNA-intercalator-topoisomerase II ternary complex
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
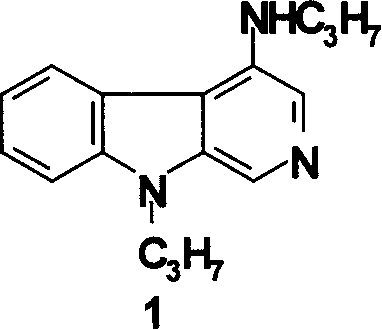
[0051] Example 1: Synthesis of compound 1 4-propylamino-9-propyl-β-carboline (1)
[0052] 1) Synthesis of 1,2,3,4-tetrahydro-β-carboline (4)
[0053]Add 20.0g of tryptamine (0.125mol) and 100mL of glacial acetic acid into a 250mL round-bottomed flask, stir at room temperature to dissolve them, add 9ml of formaldehyde solution dropwise, heat up and reflux for 1 hour after the addition, stop the reaction, cool, add 500ml of water to dilute, 2N sodium hydroxide was adjusted to make it alkaline, and a large amount of solid precipitated was filtered, washed, and dried to obtain a crude product, which was recrystallized in chloroform to obtain 415.4 g (71.6%) of the product, m.p: 209-210°C. 1 H-NMR (CDCl 3 )δ: 2.75(t, 2H, 4-CH 2 , J=5.5, 6.0Hz), 3.05(t, 2H, 3-CH 2 , J=5.5, 6.0Hz), 3.85(s, 2H, 1-CH 2 ), 7.05(t, 1H, 6-H, J=7.4, 7.7Hz), 7.10(t, 1H, 7-H, J=7.4, 8.1Hz), 7.22(d, 1H, 5-H, J= 7.7 Hz), 7.40 (d, 1H, 8-H, J = 8.1 Hz), 9.04 (m, 1H, 9-NH).
[0054] 2) Synthesis of 2-benzoy...
Embodiment 2
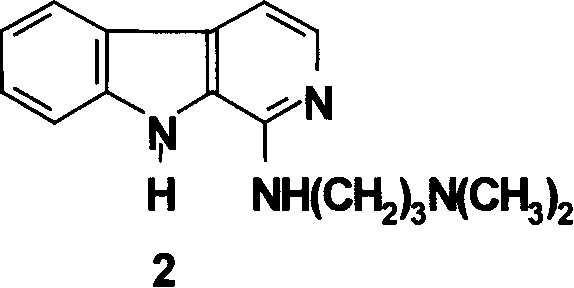
[0062] Example 2: Synthesis of compound 2 1-(3-dimethylamino)propylamino-β-carboline (2)
[0063] 1) Preparation of 2,3-diketone-3-phenylhydrazone piperidine (8)
[0064] In a 1000mL round bottom flask, add 32mL of aniline, 200mL of water and 75mL of concentrated hydrochloric acid, cool in an ice-salt bath to below minus 5°C, add dropwise a sodium nitrite / water (26g / 52mL) solution, and stir for 5 minutes after the addition is complete.
[0065] In a 2000mL round-bottomed flask, add 62g of 3-carboxylate-2-piperidone and 730mL of 3% potassium hydroxide solution, react at 25°C for 8 hours, let stand overnight at room temperature, cool to 0°C, and add diazo Saline solution, kept at 0°C and adjusted to pH 3-4 with 10% sodium bicarbonate, maintained at 0-5°C for 12 hours. The precipitated solid was filtered and washed to obtain 858.8 g (80%) of the product, m.p 243-245°C.
[0066] 2) Preparation of 1-keto-1,2,3,4-tetrahydro-β-carboline (9)
[0067] In a 250mL round bottom flask, ...
Embodiment 3
[0075] Synthesis of compound 34-methyl-3-methoxycarbonyl-1-(3-dimethylaminopropyl)carbamoyl-β-carboline (3)
[0076] 1) synthesis of ethylidene isopropylamine (12)
[0077]Add 29ml (0.34mol) of isopropylamine to a 100ml dry round bottom flask, cool in an ice-water bath, add 19ml (0.34mol) of anhydrous acetaldehyde dropwise, stir at 0°C for 0.5 hours after the addition, add 10g of potassium hydroxide, and let stand After 0.5 hours, separate the organic phase, add 5g of potassium hydroxide, let stand overnight, separate the organic phase, add 5g of potassium hydroxide, and distill to obtain b.p 60-62°C fraction 12 22g (76%).
[0078] 2) Synthesis of α-methyl-N-isopropyl-1H-indole-3-methylamine (13)
[0079] Add 3.5g of indole (30mmol) and 15ml of glacial acetic acid into a 50ml dry round bottom flask, stir to dissolve, cool in an ice-water bath, add 2.6g (30mmol) of 12 benzene solution 5ml dropwise, and stir at 0°C for 24 hours after the addition is complete , post-treatment a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com