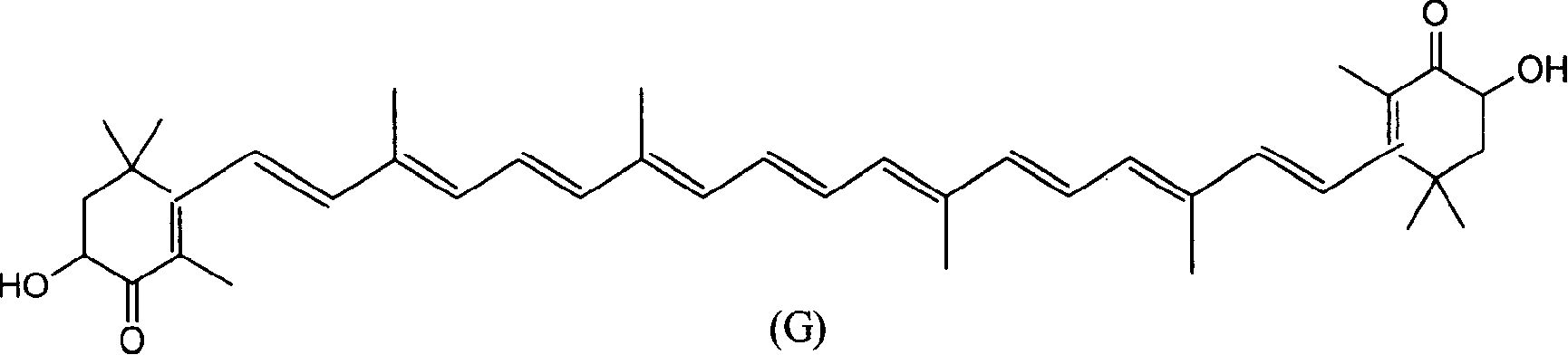
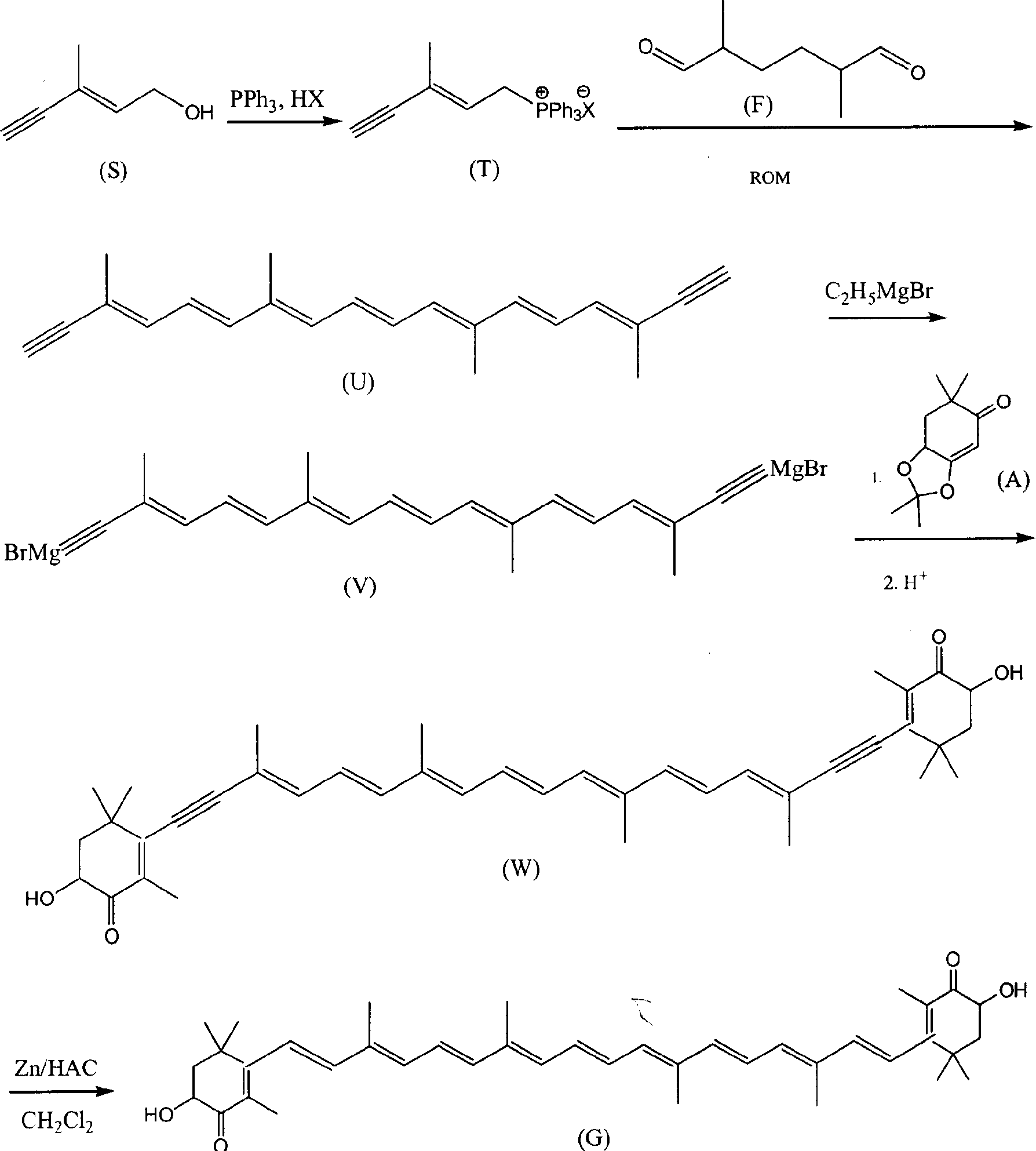
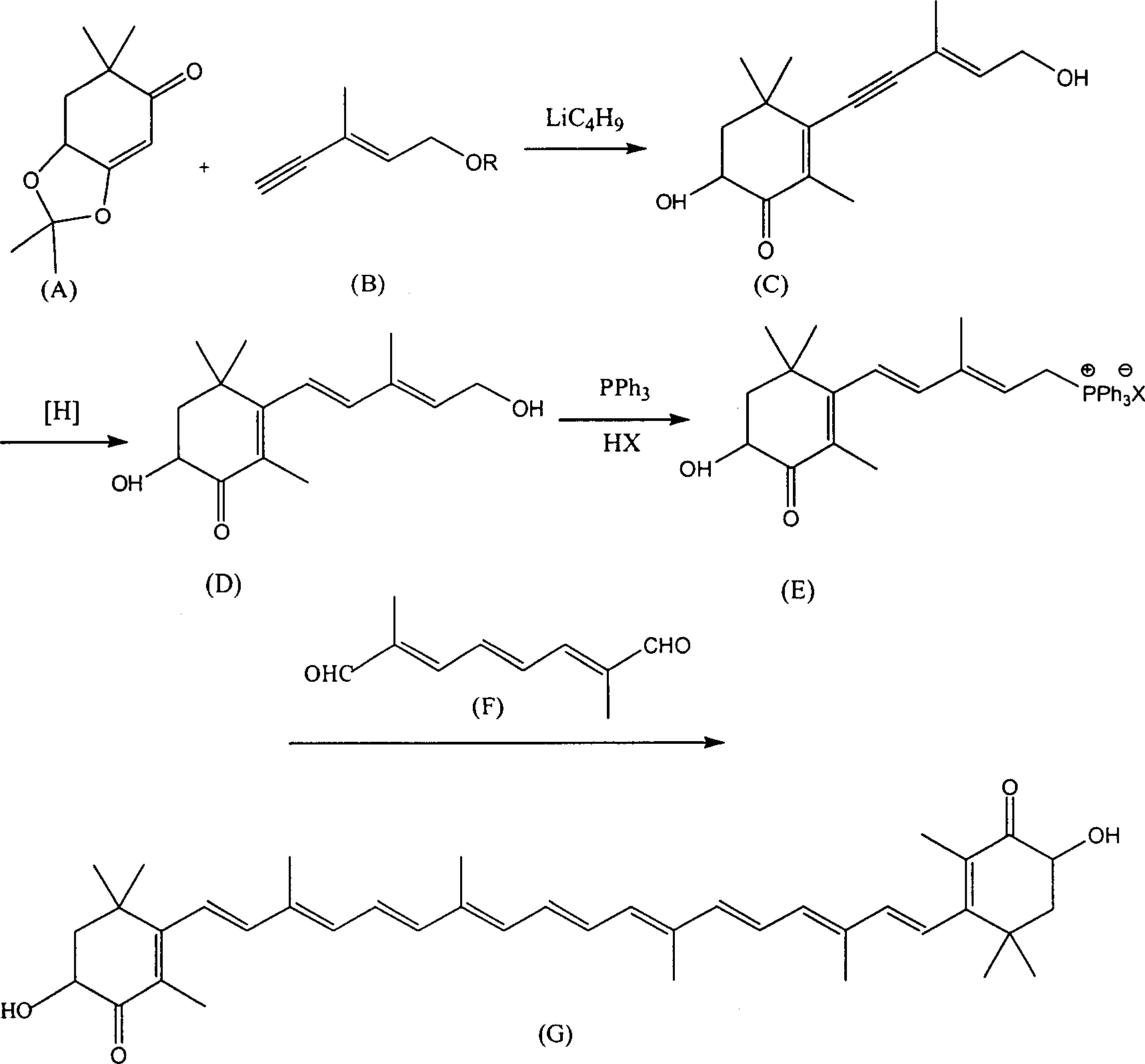
Method for synthesizing astaxsanthin
An astaxanthin and compound technology, which is applied in the field of new synthesis of astaxanthin, can solve the problems of high cost and difficult preparation of intermediates, and achieves the effects of convenient operation, low raw material cost and good reaction selectivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] a. trans C 6 Preparation of phosphonium salt (T)
[0023] Add 9.6g of C6 alcohol and 200ml of dichloromethane into a 500ml three-neck flask, add 20g of 58% hydrobromic acid dropwise at room temperature, keep the reaction for 30 minutes after dropping, add 100ml of water, and separate the dichloromethane layer. Concentrate the dichloromethane layer to 50ml, add ethyl acetate 150ml, PPh 3 28g, reacted at room temperature for 24 hours, filtered, and the filter cake was washed with 30ml ethyl acetate, dried in vacuo to obtain C 6 Phosphonium salt 26g. The mother liquor was concentrated to about 100ml, cooled, and 8g of phosphine salt was precipitated, with a total yield of 81%.
[0024] b.C 22 Preparation of Diyne Polyene Compound (U)
[0025] Add 21g of C into a 1000ml four-neck flask 6 Phosphonium salt, 4g C 10 Dialdehyde (F), 250ml of methanol, control the reaction temperature at about 10°C, add 70ml of methanol solution of 10% potassium tert-butoxide dropwise wit...
Embodiment 2
[0031] a. trans C 6 Preparation of phosphonium salt (T)
[0032] Add 9.6g of C6 alcohol and 200ml of dichloromethane into a 500ml three-neck flask, add 10g of 30% hydrochloric acid dropwise at room temperature, keep the reaction for 30 minutes after dropping, add 100ml of water, and separate the dichloromethane layer. Concentrate the dichloromethane layer to 50ml, add 150ml of methyl acetate, PPh 3 28g, reacted at room temperature for 24 hours, filtered, and the filter cake was washed with 30ml ethyl acetate, dried in vacuo to obtain C 6 Phosphonium salt 26g. The mother liquor was concentrated to about 100ml, cooled, and 7g of phosphine salt was precipitated, with a total yield of 71%.
[0033] b.C 22 Preparation of Diyne Polyene Compound (U)
[0034] Add 21g of C into a 1000ml four-neck flask 6 Phosphonium salt, 4g C 10 Dialdehyde (F), 250ml of ethanol, control the reaction temperature at about 10°C, stir and add 70ml of methanol solution of 10% potassium tert-butoxide...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com