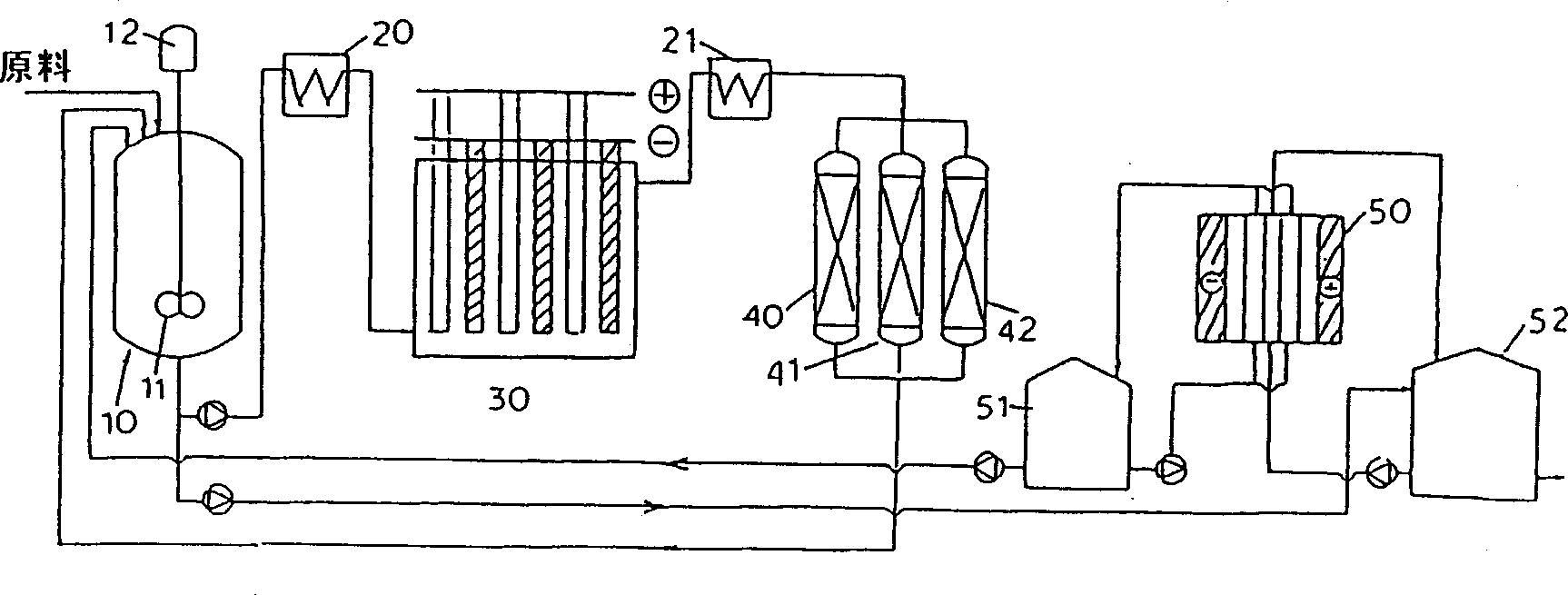
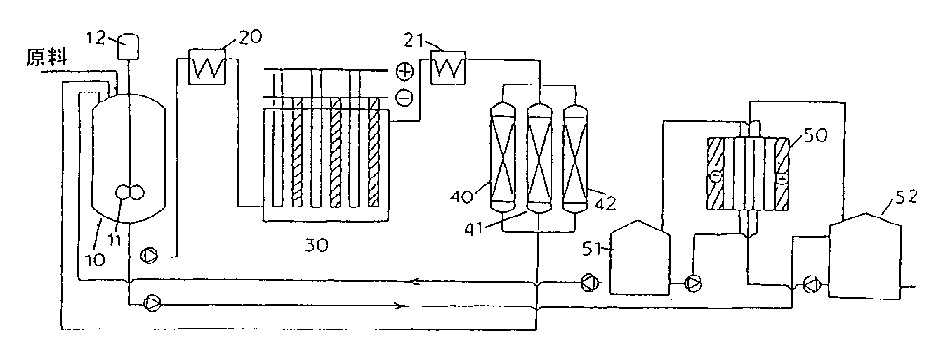
Process for selectively oxidizing primary hydroxyl group of organic compounds and resin containing adsorbed catalyst for use therein
A technology for selective oxidation of organic compounds, applied in the direction of organic compound/hydride/coordination complex catalysts, physical/chemical process catalysts, sugar compounds with non-sugar groups, etc., can solve adverse effects, inefficiencies, and undeveloped Issues such as primary hydroxyl
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] 150 mg of N-oxyl-2,2,6,6-tetramethylpiperidine (TEMPO) was added to 150 ml of water. Then 75 ml of synthetic polyacrylate resin "Diaion" HP2MG (Mitsubishi Chemical Industries) which had been previously washed and wetted with water was added thereto under stirring at room temperature. After stirring for 20 minutes, it was confirmed by gas chromatography (detection: FID, column: G-100 (40m), detection temperature: 150°C) that TEMPO in the aqueous solution was adsorbed by the resin at a rate of 98.0% or more. The resin with TEMPO adsorbed thereon was then filtered off to obtain about 75 ml of resin with TEMPO adsorbed thereon.
Embodiment 2
[0069]Dissolve 9.7 g of methyl-α-D-glucopyranoside in 150 ml of water. Under stirring, 5.3 g of anhydrous sodium carbonate, 2.0 g of sodium bromide and 75 ml of the TEMPO-adsorbed resin obtained in Example 1 above were added. The resulting mixture was kept at an internal temperature of 30°C or lower with stirring, and about 190 ml of an aqueous sodium hypochlorite solution containing 5% active chlorine was added dropwise. After stirring for 1.5 hours, by HPLC (detection: RI, UV 210 nm, column: Shodex SUGAR SH1011, column temperature: 25°C, mobile phase: 0.1% phosphoric acid aqueous solution; or RI, UV 210 nm, column: Aminex HPX-87H, Column temperature: 60°C, mobile phase: 0.1N sulfuric acid) proved that methyl-α-D-glucopyranoside had been converted into methyl-α-D-glucopyranoside uronic acid at a rate of 100%. After the above reaction was completed, it was confirmed by gas chromatography (detection: FID, column: G-100 (40m), detection temperature: 150° C.) that no TEMPO was e...
Embodiment 3
[0071] Dissolve 9.7 g of methyl-α-D-glucopyranoside in 150 ml of water. Under stirring, 8.0 g of anhydrous sodium carbonate, 2.0 g of sodium bromide and 150 ml of TEMPO were added. While maintaining the resulting mixture at an internal temperature of 30°C or lower with vigorous stirring, about 60 ml of an aqueous sodium hypochlorite solution containing 12% active chlorine was added dropwise. After stirring for 1.5 hours, it was proved by HPLC (detection: RI, UV 210 nm, column: Shodex SUGAR SH1011, column temperature: 25°C, mobile phase: 0.1% phosphoric acid aqueous solution) that methyl-α-D-glucopyranoside had been A rate of 100% was converted to methyl-α-D-glucopyranoside uronic acid. Subsequently, 75 ml of synthetic polyacrylate resin "Diaion" HP2MG (Mitsubishi Chemical Industries), which had been previously washed and wetted with water, was directly added to the liquid reaction mixture under stirring at room temperature, thereby allowing the resin to adsorb TEMPO in the li...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com