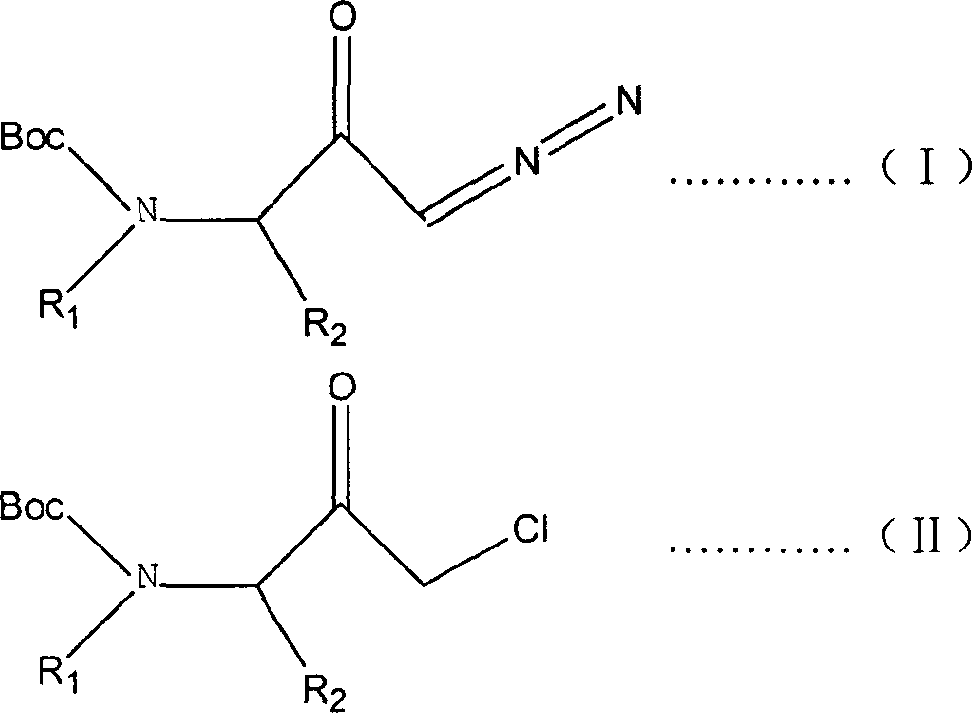
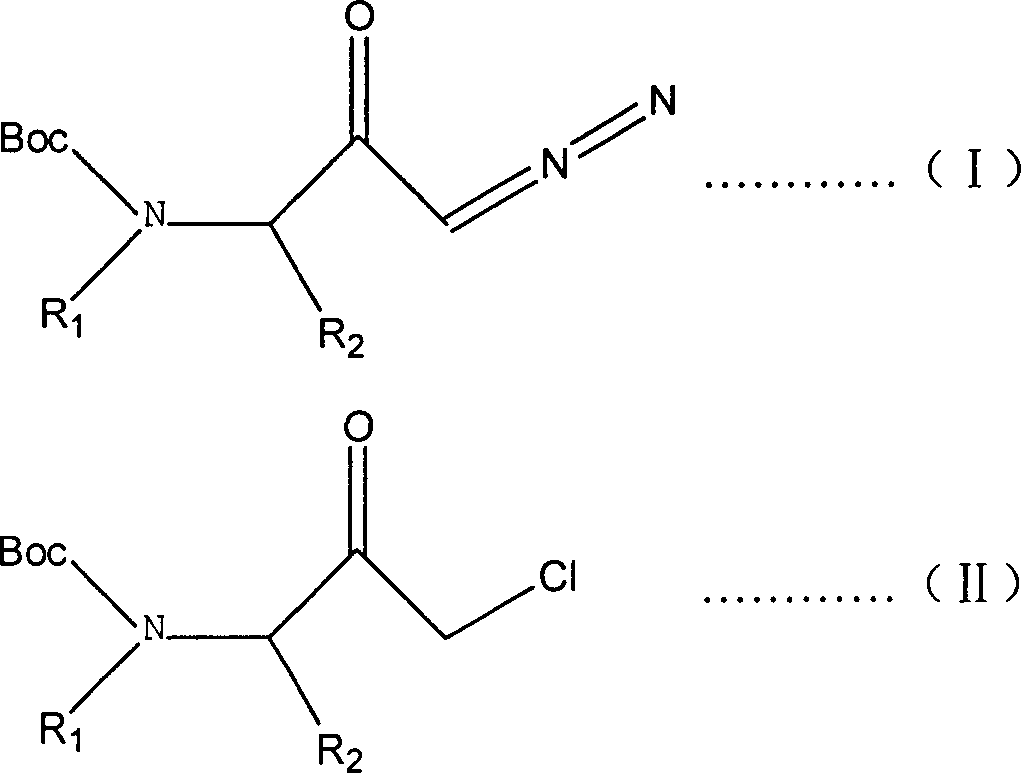
Prepn process of 1-chloro-3-(N-substituent)-tert-butyloxy formylamido-3-substituent-2-acetone
A technology of tert-butoxycarboxamido and acetone is applied in the field of preparing 1-chloro-3--tert-butoxycarboxamido-3-substituted-2-acetone, which can solve the industrial pollution of metal cations and increase the burden of treatment of three wastes , the problem of high price of metal chlorides, to achieve the effect of environmental friendliness, low burden on the three wastes treatment, and low prices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] In the laboratory, 35mmol of (3S)-1-diazo-3-tert-butoxycarboxamido-3-methyl-2-propanone was dissolved in a mixed solvent composed of 100ml tetrahydrofuran and 200ml ether, and then the above mixed solution was placed Put it into a 500ml three-necked flask, feed dry 35mmol hydrogen chloride gas under the protection of argon at 0°C, and spin the solvent under reduced pressure to obtain 6.4 g of the desired product as a white solid, with a yield of 83%. Structural identification of the target product, which 1 H nuclear magnetic resonance spectrometer 1 H-NMR (600MHZ, CDCl 3 ) data: δ 1.37 (d, 3H), 1.44 (s, 9H), 4.26 (m, 2H), 5.08 (s, 1H). [α] D 20 = -48.3 (c = 1.0, EtOH). The product was shown to be (3S)-1-chloro-3-tert-butoxycarboxamido-3-methyl-2-propanone.
Embodiment 2
[0019] In the laboratory, 48mmol (3S)-1-diazo-3-tert-butoxycarboxamido-3-(2'-methylpropyl)-2-propanone was dissolved in a mixed solvent composed of 100ml tetrahydrofuran and 200ml ether, Then put the above mixed solution into a 500ml three-neck flask, pass through dry 96mmol hydrogen chloride gas at 10°C under the protection of neon gas, and spin the solvent under reduced pressure to obtain 11.8 grams of the target product as a white solid with a yield of 93%. Structural identification of the target product, which 1 H nuclear magnetic resonance spectrometer 1 H-NMR (600MHZ, CDCl 3 ) data: δ0.9(m, 3H), 0.96(d, 1H), 1.10(m, 3H), 1.14(m, 1H), 1.37(m, 1H), 1.44(s, 9H), 1.90(m , 1H), 4.24(m, 2H), 4.40(m, 1H), 5.0(d, 1H). [α] D 20 = -37.1 (c = 1.0, EtOH). The product was shown to be (3S)-1-chloro-3-tert-butoxycarboxamido-3-(2'-methylpropyl)-2-propanone.
Embodiment 3
[0021] Dissolve 76mmol (±)-1-diazo-3-tert-butoxycarboxamido-3-benzyl-2-propanone in a mixed solvent composed of 100ml tetrahydrofuran and 200ml 1,4-dioxane , and then put the above mixed solution into a 500ml three-necked flask, pass through dry 228mmol hydrogen chloride gas at 25°C under the protection of argon, and spin the solvent under reduced pressure to obtain 21.0 grams of white solid, with a yield of 92.5%. Structural identification of the target product, which 1 H nuclear magnetic resonance spectrometer 1 H-NMR (300MHZ, CDCl 3 ) data: δ1.40 (s, 9H), 3.0-3.1 (m, 2H), 3.9-4.2 (dd, 2H), 4.65 (m, 1H), 5.0 (d, 1H), 7.15-7.24 (d, 2H), 7.3 (m, 3H). [α] D 20 =0 (c=1.0, EtOH). The product was shown to be (±)-1-chloro-3-tert-butoxycarboxamido-3-benzyl-2-propanone.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com