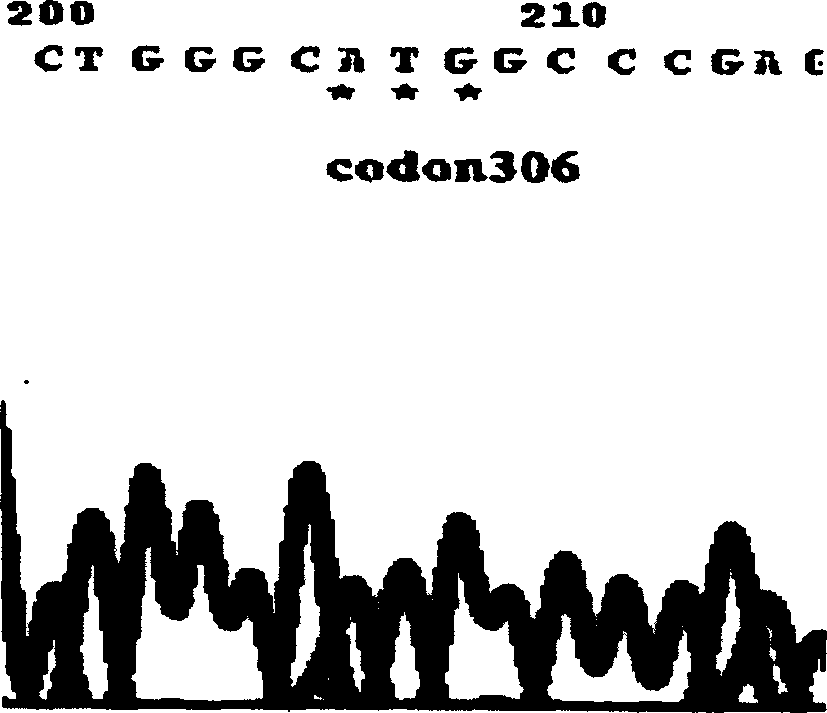
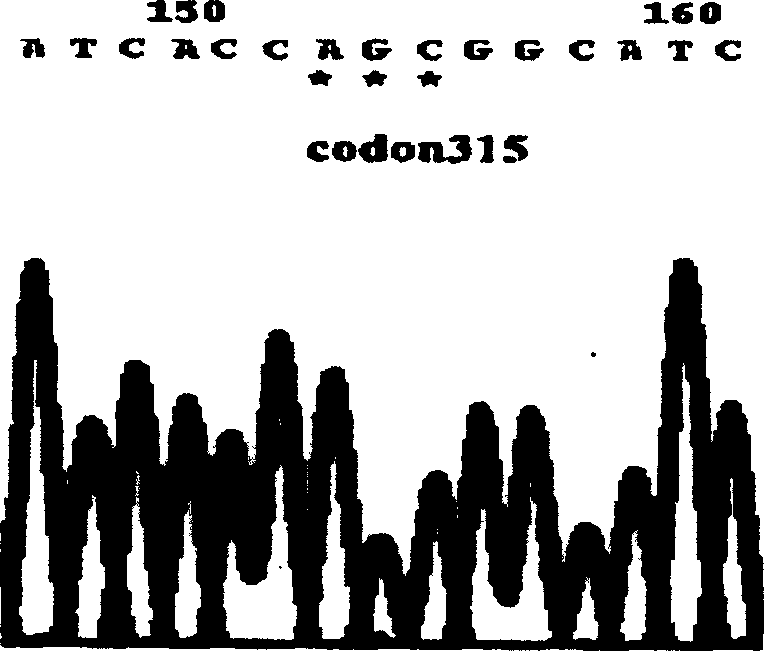M.tuberculosis drug resistant gene detection reagent kit and process for preparation
A technology of Mycobacterium tuberculosis and a detection kit, which is applied in the field of medical molecular biology diagnosis, and can solve the problems of expensive sequencing equipment, high cost, expensive spotting system and fluorescent analysis system, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] A preparation method of a Mycobacterium tuberculosis drug-resistant gene detection kit, comprising the following steps:
[0071] 1. The preparation method of the Mycobacterium tuberculosis resistance gene detection patch is as follows:
[0072] Probe code
No
a
Sequence (5'→3')
M
16S rRNA
5'-TATTAGACCCAGTTTCCC
MT
16S rRNA
5'-ACAAGACATGCATCCCGT-3'
No.1
rpoB 531
5′-CC CAG CGC CGA CAG TC-3'
wild type (TCG)
No.1b
rpoB 531
5′--A GCG C CA A CA GTC GG-3'
mutant (TTG)
No.1c
rpoB 531
5′--CCA GCG C CC A CA GTC-3'
mutant (TGG)
No.1d
rpoB 531
5′--CCA GCG C GT A CA GTC G-3'
mutant (TAC)
No.2
rpoB 526
5′-GCG CTT GTG GGT CAA-3'
wild type (CAC)
No.2b
rpoB 526
5′-G GCG CTT GTA GGT CAA C-3'
mutant (TAC)
No.2c
rpoB 526
5′-G GCG CTT GTC GGT CA-3'
mutant (G...
Embodiment 2
[0123] A preparation method of a Mycobacterium tuberculosis drug-resistant gene detection kit, comprising the following steps:
[0124] 1. The preparation method of the Mycobacterium tuberculosis resistance gene detection patch is as follows:
[0125] Probe code
No
a
Sequence (5'→3')
M
16S rRNA
5'-TATTAGACCCAGTTTCCC
MT
16S rRNA
5'-ACAAGACATGCATCCCGT-3'
No.1
rpoB531
5′-CC CAG CGC CGA CAG TC-3'
wild type (TCG)
No.1b
rpoB 531
5′--A GCG C CA A CA GTC GG-3'
mutant (TTG)
No.1c
rpoB 531
5′--CCA GCG C CC A CA GTC-3'
mutant (TGG)
No.1d
rpoB 531
5′--CCA GCG C GT A CA GTC G-3'
mutant (TAC)
No.2
rpoB 526
5′-GCG CTT GTG GGT CAA-3'
wild type (CAC)
No.2b
rpoB 526
5′-G GCG CTT GTA GGT CAA C-3'
mutant (TAC)
No.2c
rpoB 526
5′-G GCG CTT GTC GGT CA-3'
muta...
Embodiment 3
[0175] A preparation method of a Mycobacterium tuberculosis drug-resistant gene detection kit, comprising the following steps:
[0176] 1. The preparation method of the Mycobacterium tuberculosis resistance gene detection patch is as follows:
[0177] Probe code
No
a
Sequence (5'→3')
M
16S rRNA
5'-TATTAGACCCAGTTTCCC
MT
16S rRNA
5'-ACAAGACATGCATCCCGT-3'
No.1
rpoB531
5′-CC CAG CGC CGA CAG TC-3'
wild type (TCG)
No.1b
rpoB 531
5′--A GCG C CA A CA GTC GG-3'
mutant (TTG)
No.1c
rpoB 531
5′--CCA GCG C CC A CA GTC-3'
mutant (TGG)
No.1d
rpoB 531
5′--CCA GCG C GT A CA GTC G-3'
mutant (TAC)
No.2
rpoB 526
5′-GCG CTT GTG GGT CAA-3'
wild type (CAC)
No.2b
rpoB 526
5′-G GCG CTT GTA GGT CAA C-3'
mutant (TAC)
No.2c
rpoB 526
5′-G GCG CTT GTC GGT CA-3'
mutant (GA...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com